

Original Article - DOI:10.33594/000000576
Accepted 2 October 2022 - Published online 19 October 2022
1Department of Veterinary and Animal Sciences, University of Rajshahi, Rajshahi, Bangladesh;
2Department of Physiology, University of Tübingen, Tübingen, Germany;
3Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany;
4Zebrafish Neuromorphology Lab, Department of Veterinary Sciences, University of Messina, Messina, Italy;
5Department of Biological and Environmental Sciences, University of Messina, Messina, Italy
Background/Aims: Pentostatin (2'-deoxycoformycin), a purine analog, is used for the treatment of diverse B and T-cell malignancies as well as for immunosuppression. Pentostatin is at least in part effective by triggering apoptosis. Pentostatin sensitive mechanisms leading to apoptosis include accumulation of DNA strand breaks, altered transcription and mitochondrial depolarization. Erythrocytes lack nuclei and mitochondria but nevertheless may enter eryptosis, an apoptosis-like suicidal cell death characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the erythrocyte surface. Stimulators of eryptosis include increase of cytosolic Ca2+-activity ([Ca2+]i), ceramide formation and energy depletion. The present study tested, whether and how pentostatin induces eryptosis. Methods: The phosphatidylserine exposure at the cell surface was estimated from annexin V binding, cell volume from forward scatter, hemolysis from hemoglobin release, [Ca2+]i from Fluo3-fluorescence, ceramide abundance utilizing specific antibodies, and cytosolic ATP concentration utilizing a luciferin–luciferase assay kit. Results: A 48 hours exposure of human erythrocytes to pentostatin (≥5 µg/ml) significantly increased the percentage of annexin-V-binding cells and significantly decreased forward scatter. Pentostatin significantly increased [Ca2+]i, and significantly decreased cytosolic ATP, but did not significantly modify ceramide abundance. The effect of pentostatin on annexin-V-binding was significantly blunted, but not abolished by removal of extracellular Ca2+. Conclusion: Pentostatin triggers erythrocyte shrinkage and phospholipid scrambling of the erythrocyte cell membrane, effects paralleled by and at least partially due to entry of extracellular Ca2+ and cellular energy depletion.
Pentostatin (2'-deoxycoformycin), a purine analog and specific adenosine deaminase inhibitor [1-6], is effective against a variety of B- and T-cell malignancies [6-11] including hairy cell leukemia [1-3, 12-17], chronic lymphocytic leukemia [1, 4, 18, 19], lymphoplasmacytic lymphoma [8], cutaneous T-cell lymphoma [20], low grade non-Hodgkin's lymphoma [5], and autoimmune lymphoproliferative syndrome [21]. Moreover, the drug has been used as immunosuppressant [22, 23] and treatment of graft-versus-host disease [23, 24]. Pentostatin has further antiviral and antimalarial potency, and protects CNS cells against ischemic and hypoxic injury [5].
Pentostatin is at least in part effective by triggering apoptosis [3, 11, 24]. Pentostatin sensitive cellular mechanisms include accumulation of DNA strand breaks [3], activation of transcription factor p53 [3], release of cytochrome c from mitochondria, [3], activation of poly(ADP-ribose) polymerase (PARP) [3], and cellular depletion of nicotinamide adenine dinucleotide (NAD) and ATP [3].
Even though lacking nuclei and mitochondria, erythrocytes may enter eryptosis, the suicidal erythrocyte death characterized by cell shrinkage [25] and phospholipid scrambling of the cell membrane with phosphatidylserine translocation to the cell surface [26]. Cellular mechanisms involved in the stimulation of eryptosis include oxidative stress [26], Ca2+ entry with increase of cytosolic Ca2+ activity ([Ca2+]i) [26], ceramide [27], energy depletion [26], activated caspases [26, 28, 29], enhanced activity of casein kinase 1α, Janus-activated kinase JAK3, protein kinase C, p38 kinase and PAK2 kinase [26], as well as decreased activity of AMP activated kinase AMPK, cGMP-dependent protein kinase, and sorafenib/sunitinib sensitive kinases [26].
The present study explored, whether and how pentostatin may stimulate eryptosis. To this end phosphatidylserine surface abundance and cell volume were determined by flow cytometry in human erythrocytes from healthy volunteers without and with prior exposure to pentostatin. Additional experiments addressed the influence of pentostation on [Ca2+]i, ceramide abundance ands cyotsolic ATP levels.
Erythrocytes, solutions and chemicalsFresh Li-Heparin-anticoagulated blood samples were kindly provided by the blood bank of the University of Tübingen. The study is approved by the ethics committee of the University of Tübingen (184/2003 V). The blood was centrifuged at 120 g for 20 min at 21°C and the platelets and leukocytes-containing supernatant was disposed. Erythrocytes were incubated in vitro at a hematocrit of 0.4% in Ringer solution containing (in mM) 5 glucose, 1 CaCl2, 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), pH 7.4 at 37°C for 48 h. Where indicated, erythrocytes were exposed to pentostatin (Sigma Aldrich, Hamburg, Germany) at the indicated concentrations.
Determination of annexin-V-binding and forward scatterAfter incubation under the respective experimental condition, 150 µl cell suspension was washed in Ringer solution containing 5 mM CaCl2 and then stained with Annexin-V-FITC (1:200 dilution; ImmunoTools, Friesoythe, Germany) in this solution at 37°C for 20 min under protection from light. The annexin V abundance at the erythrocyte surface was subsequently determined on a FACS Calibur (BD, Heidelberg, Germany). A dot plot of forward scatter (FSC) vs. side scatter (SSC) was set to linear scale for both parameters. The threshold of forward scatter was set at the default value of “52”. The cells were analyzed by flow cytometry with an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Measurement of hemolysisFor the determination of hemolysis, the samples were centrifuged (3 min at 1600 rpm, room temperature) after incubation under the respective experimental conditions and the supernatants were harvested. As a measure of hemolysis, the hemoglobin (Hb) concentration of the supernatant was determined photometrically at 405 nm. The absorption of the supernatant of erythrocytes lysed in distilled water was defined as 100% hemolysis.
Determination of intracellular Ca2+After incubation, erythrocytes were washed in Ringer solution and then loaded with Fluo-3/AM (Biotium, Hayward, USA) in Ringer solution containing 5 mM CaCl2 and 5 µM Fluo-3/AM. The cells were incubated at 37°C for 30 min and washed twice in Ringer solution containing 5 mM CaCl2. The Fluo-3/AM-loaded erythrocytes were resuspended in 200 µl Ringer. Then, Ca2+-dependent fluorescence intensity was measured with an excitation wavelength of 488 nm and an emission wavelength of 530 nm on a FACS Calibur and the threshold was set at the default value of “52”.
Determination of ceramide formationFor the determination of ceramide, a monoclonal antibody-based assay was used. After incubation, cells were stained for 1 hour at 37°C with 1 µg/ml anti ceramide antibody (clone MID 15B4, Alexis, Grünberg, Germany) in PBS containing 0.1% bovine serum albumin (BSA) at a dilution of 1:10. The samples were washed twice with PBS-BSA. Subsequently, the cells were stained for 30 minutes with polyclonal fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG and IgM specific antibody (Pharmingen, Hamburg, Germany) diluted 1:50 in PBS-BSA. Unbound secondary antibody was removed by repeated washing with PBS-BSA. The samples were then analyzed by flow cytometric analysis with an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Determination of intracellular ATP concentrationFor the determination of intracellular erythrocyte ATP, 90 µl of erythrocyte pellets were incubated for 48 h at 37°C in Ringer solution with or without pentostatin (10 µg/ml). All subsequent manipulations were performed at 4°C to avoid ATP degradation. Cells were lysed in distilled water, and proteins were precipitated by addition of HClO4 (5%). After centrifugation, an aliquot of the supernatant (400 µl) was adjusted to pH 7.7 by addition of saturated KHCO3 solution. After dilution of the supernatant, the ATP concentrations of the aliquots were determined utilizing a luciferin–luciferase assay kit (Roche Diagnostics) on a luminometer (Berthold Biolumat LB9500, Bad Wildbad, Germany) according to the manufacturer’s protocol. ATP concentrations are expressed in mmol/l cytosol of erythrocytes.
StatisticsData are expressed as arithmetic means ± SEM. As indicated in the figure legends, statistical analysis was made using ANOVA with Tukey’s test as post-test and t test as appropriate. n denotes the number of different erythrocyte specimens studied. Since different erythrocyte specimens used in distinct experiments are differently susceptible to triggers of eryptosis, the same erythrocyte specimens have been used for control and experimental conditions.
The present study tested, whether pentostatin stimulates suicidal erythrocyte death or eryptosis. Hallmarks of eryptosis include cell membrane scrambling with phosphatidylserine translocation to the cell surface. Phosphatidylserine exposing erythrocytes were identified utilizing annexin-V-binding, as determined by flow cytometry. The erythrocytes were analysed following incubation for 48 hours in Ringer solution without or with pentostatin (0.5 - 10 µg/ml). As illustrated in Fig. 1, a 48 hours exposure to pentostatin increased the percentage of annexin-V-binding, an effect reaching statistical significance at 5 µg/ml pentostatin concentration.
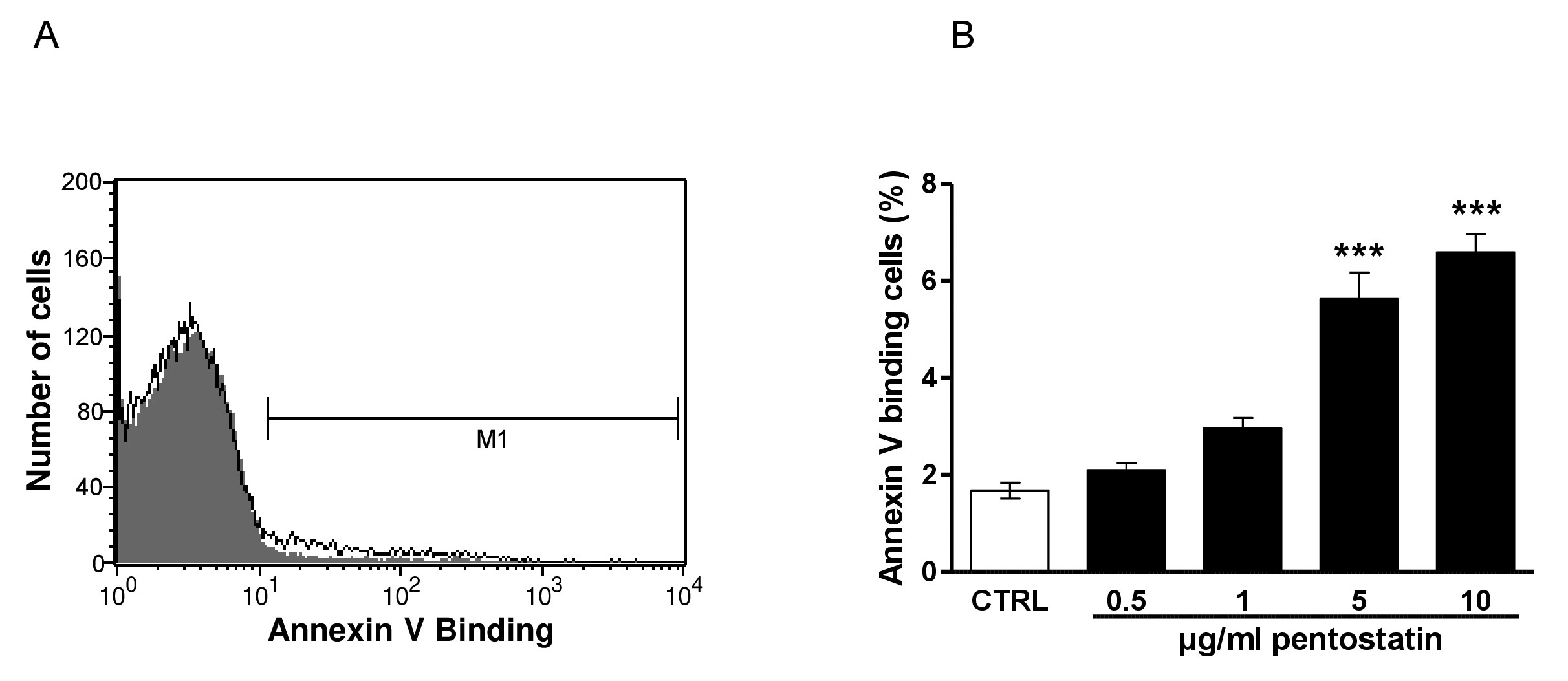
Fig. 1. Effect of pentostatin on phosphatidylserine exposure. A. Original histogram of annexin-V-binding of erythrocytes following exposure for 48 hours to Ringer solution without (grey area) and with (black line) presence of 10 µg/ml pentostatin. The marker M1 indicates the annexin-V positive erythrocytes. B. Arithmetic means ± SEM (n = 12) of erythrocyte annexin-V-binding (black bars) following incubation for 48 hours to Ringer solution without or with presence of pentostatin (0.5 - 10 µg/ml). ***(p<0.001) indicates significant difference from the absence of pentostatin (ANOVA).
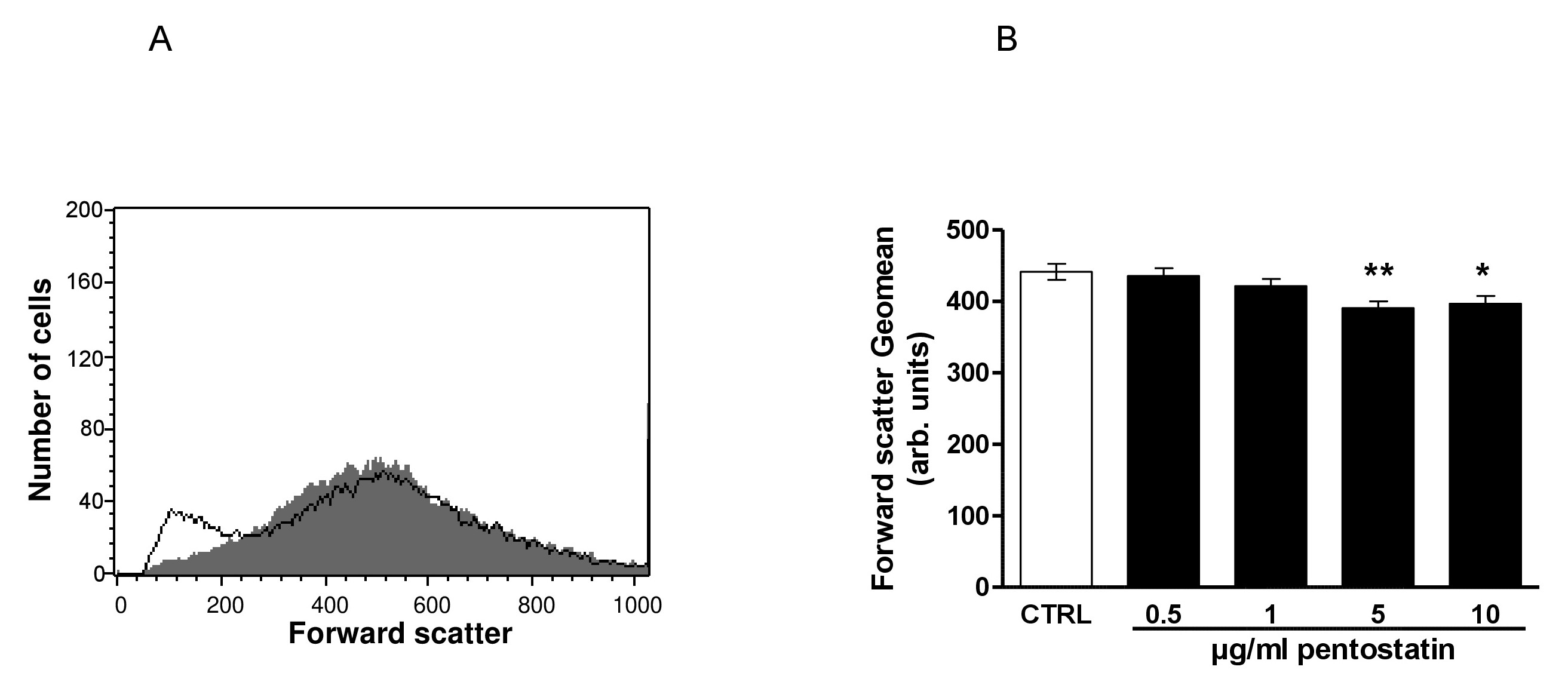
Fig. 2. Effect of pentostatin on erythrocyte forward scatter. A. Original histogram of forward scatter of erythrocytes following exposure for 48 hours to Ringer solution without (grey area) and with (black line) presence of 10 µg/ml pentostatin. B. Arithmetic means ± SEM (n = 12) of the erythrocyte forward scatter (FSC) following incubation for 48 hours to Ringer solution without (white bar) or with (black bars) pentostatin (0.5 - 10 µg/ml). *(p<0.05), **(p<0.01) indicate significant difference from the absence of pentostatin (ANOVA).
In order to quantify hemolysis, the hemoglobin concentration was determined in the supernatant by photometry. As a result, the percentage of hemolytic erythrocytes was after a 48 hours incubation similar in the absence of pentostatin (1.29 ± 0.42, n = 12) and in the presence of 0.5 µg/ml (1.24 ± 0.45, n = 12), 1 µg/ml (1.12 ± 0.29, n = 12), 5 µg/ml (1.07 ± 0.26, n = 12) and 10 µg/ml (1.36 ± 0.37, n = 12) pentostatin. Thus, pentostatin (0.5 -10 µg/ml) did not significantly induce hemolysis.
The second hallmark of eryptosis is cell shrinkage. In order to estimate cell volume, forward scatter was determined utilizing flow cytometry following a 48 hours incubation in Ringer solution without or with pentostatin (0.5 – 10 µg/ml). As shown in Fig. 2, pentostatin decreased erythrocyte forward scatter, an effect reaching statistical significance at 5 µg/ml pentostatin concentration.
In order to determine, whether pentostatin modifies cytosolic Ca2+ activity ([Ca2+]i), Fluo3 fluorescence was taken as measure of [Ca2+]i. As shown in Fig. 3, a 48 hours exposure to pentostatin increased the Fluo3 fluorescence, an effect reaching statistical significance at 5 µg/ml pentostatin concentration.
A next series of experiments explored whether pentostatin-induced translocation of phosphatidylserine or erythrocyte shrinkage required entry of extracellular Ca2+. To this end, erythrocytes were incubated for 48 hours in the absence or presence of 10 µg/ml pentostatin in the presence or nominal absence of extracellular Ca2+. As illustrated in Fig. 4, removal of extracellular Ca2+ significantly blunted the effect of pentostatin on annexin-V-binding. Thus, pentostatin induced cell membrane scrambling was in large part due to entry of extracellular Ca2+.
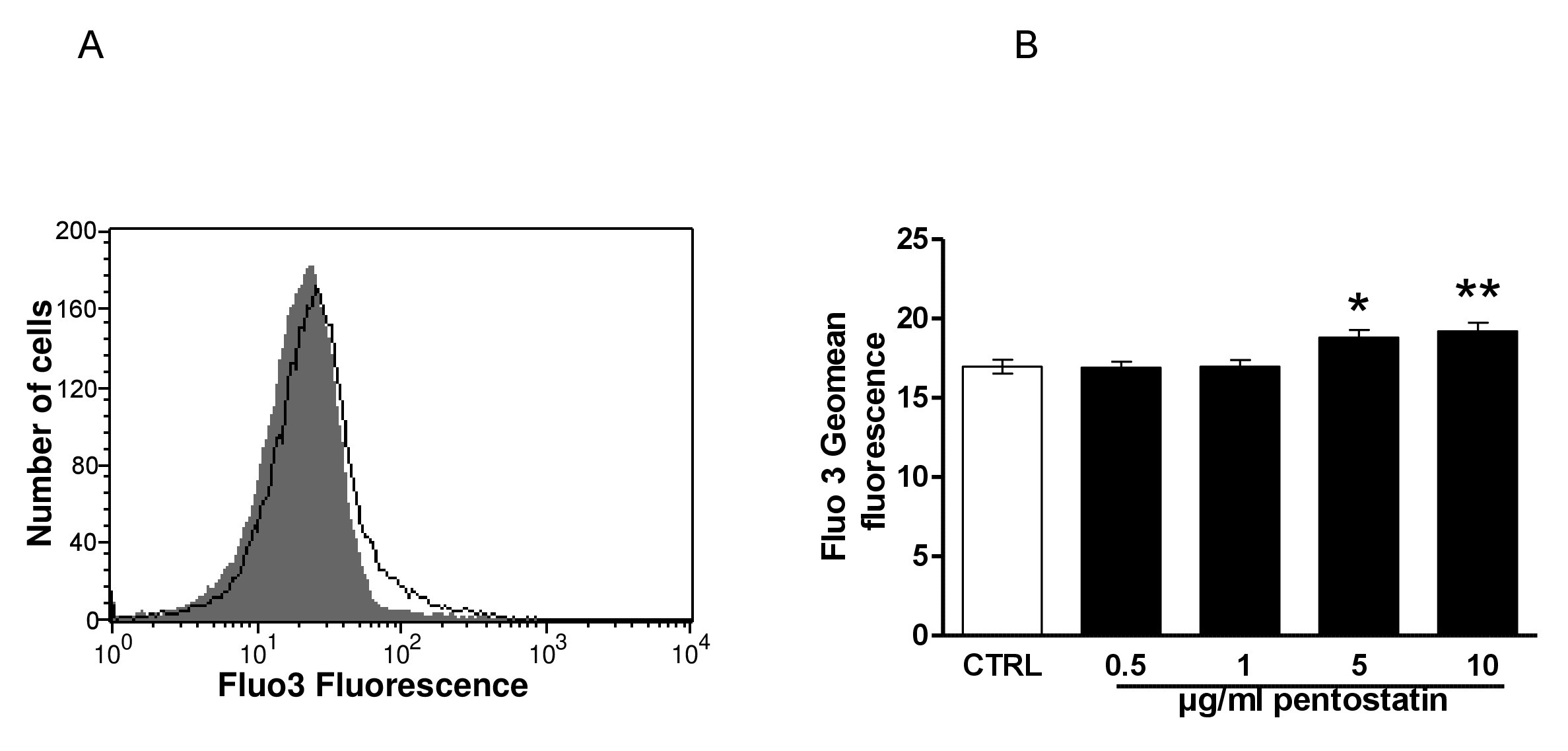
Fig. 3. Effect of pentostatin on erythrocyte Ca2+ activity. A. Original histogram of Fluo3 fluorescence in erythrocytes following exposure for 48 hours to Ringer solution without (grey area) and with (black line) presence of pentostatin (10 µg/ml). B. Arithmetic means ± SEM (n = 20) of the Fluo3 fluorescence (arbitrary units) in erythrocytes exposed for 48 hours to Ringer solution without (white bar) or with (black bars) pentostatin (0.5 – 10 µg/ml). *(p<0.05) **(p<0.01) indicate significant difference from the absence of pentostatin (ANOVA).
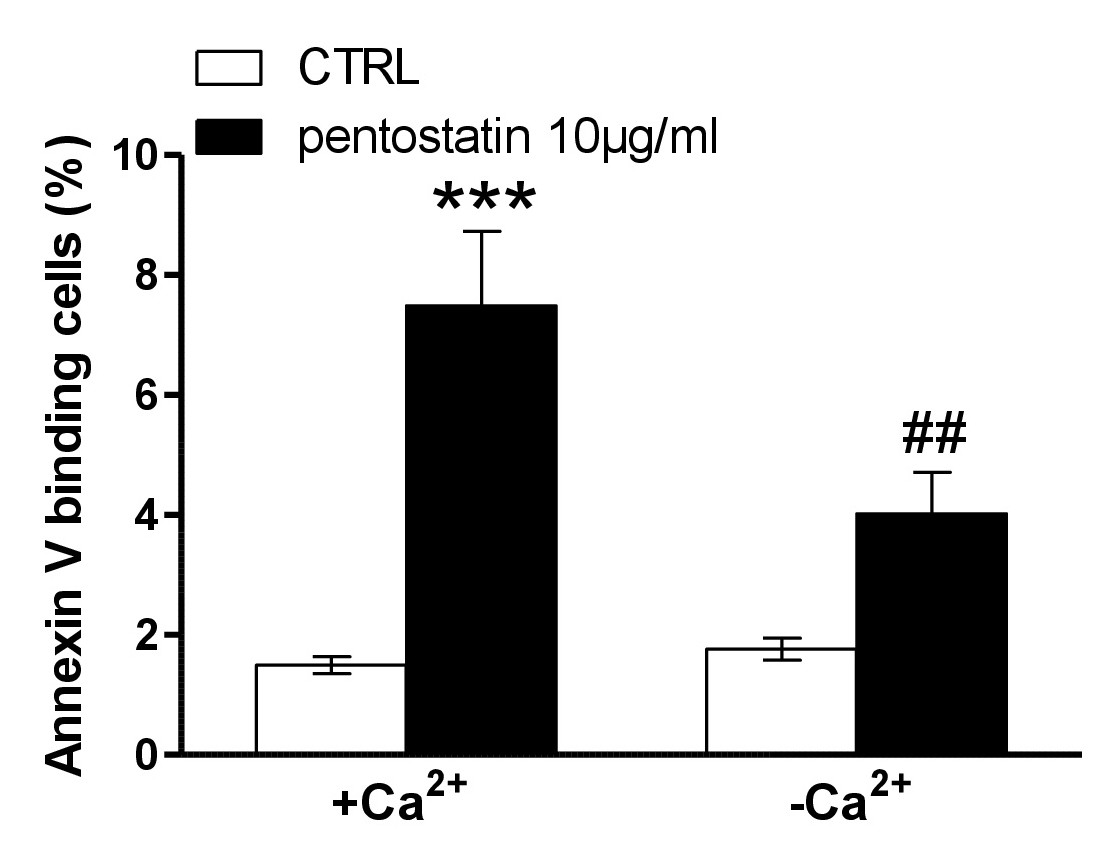
Fig. 4. Ca2+ sensitivity of pentostatin-induced phosphatidylserine exposure. A, B. Original histogram of annexin-V-binding of erythrocytes following exposure for 48 hours to Ringer solution without (grey area) and with (black line) presence of pentostatin (10 µg/ml) in the presence (A) and absence (B) of extracellular Ca2+. Arithmetic means ± SEM (n = 11) of annexin-V-binding of erythrocytes after a 48 hours treatment with Ringer solution without (white bars) or with (black bars) pentostatin (10 µg/ml) in the presence (left bars, +Ca2+) and absence (right bars, -Ca2+) of Ca2+. ***(P<0.001) indicates significant difference from the absence of pentostatin, ##(p<0.01) indicates significant difference from the presence of Ca2+ (ANOVA).
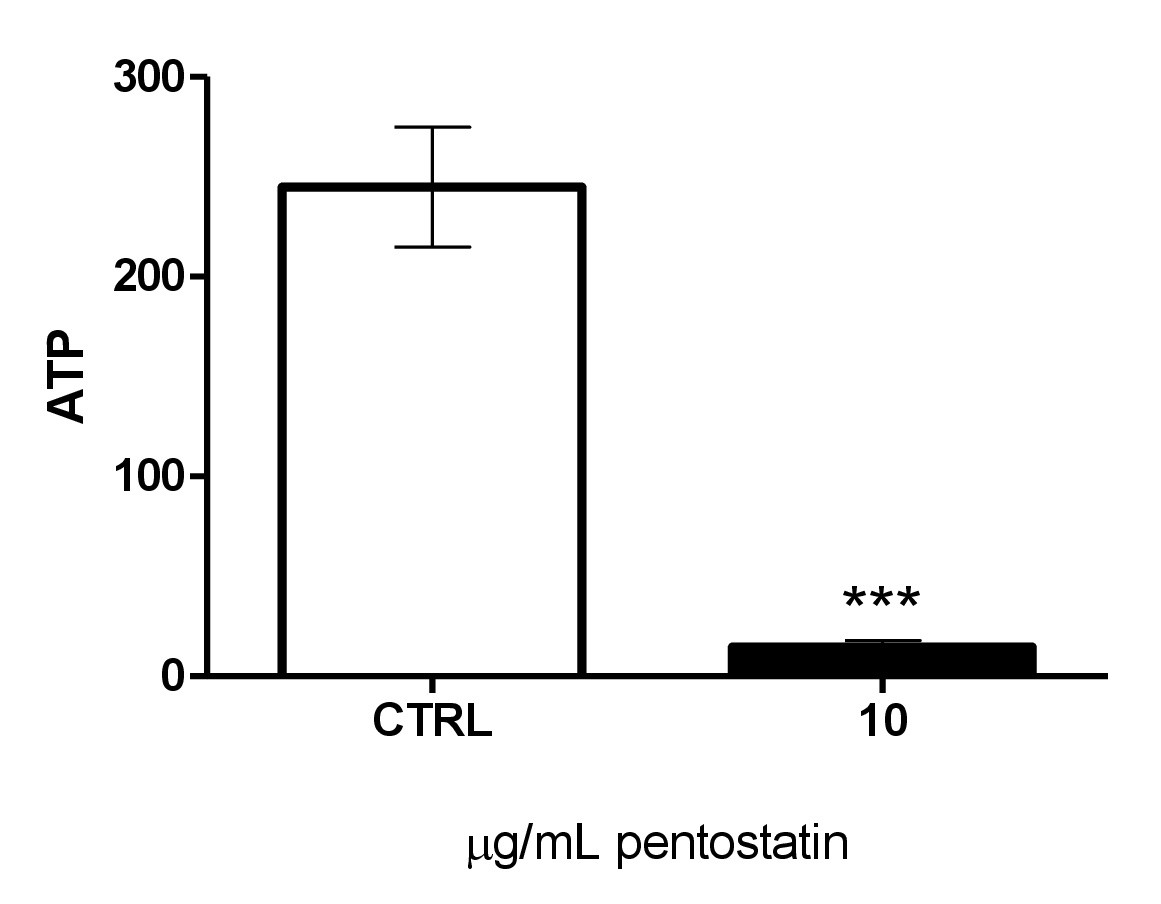
Fig. 5. Effect of pentostatin on erythrocyte ATP levels. Arithmetic means ± SEM (n = 4) of cellular ATP (arbitrary units) in erythrocytes exposed for 48 hours to Ringer solution without (white bar) or with (black bar) pentostatin (10 µg/ml). ***(p<0.001) indicates significant difference from the absence of pentostatin (ANOVA).
Stimulators of eryptosis in the absence of increased [Ca2+]i include ceramide. Thus, specific antibodies were utilized to quantify ceramide abundance at the erythrocyte surface. As a result, the percentage of ceramide exposed erythrocytes was similar following a 48 hours incubation in the absence (12.76 ± 0.94, n = 11) and presence (13.65 ± 1.16, n = 11) of 10 µg/ml pentostatin.
Eryptosis is further stimulated by energy depletion. A luciferin–luciferase assay was employed to determine the cytosolic ATP level. As illustrated in Fig. 5, a 48 hours exposure to 10 µg/ml significantly decreased the cytosolic ATP concentration.
The present observations reveal a novel effect of pentostatin, i.e. the triggering of erythrocyte death or eryptosis. Pentostatin treatment of human erythrocytes drawn from healthy individuals is followed by cell shrinkage and phosphatidylserine translocation to the erythrocyte surface, the two hallmarks of eryptosis [26]. The pentostatin concentration (5 µg/ml) required for statistically significant stimulation of erythrocyte cell membrane scrambling was higher than the plasma concentrations of 0.5 µg/ml or 0.78 µg/ml [30] observed in patients. Moreover, in vivo, the pentostatin concentration may be lowered by plasma protein binding [30, 31]. Thus, pentostatin may induce eryptosis only after pentostatin intoxicaton rather than at therapeutic dosage. On the other hand, the possibility must be kept in mind that the susceptibility to triggers of eryptosis is enhanced by eryptosis-inducing clinical disorders. Eryptosis is induced by a wide variety of xenobiotics [26] and in several clinical conditions including dehydration [32], hyperphosphatemia [33] chronic kidney disease (CKD) [34-37], Hemolytic-uremic syndrome [38], diabetes [39], liver failure [40], malignancy [41], sepsis [42] Sickle-cell disease [40], beta-thalassemia [40], Hb-C [40], G6PD-deficiency [40] and Wilsons disease [43].
The effects of pentostatin on cell membrane scrambling and cell shrinkage was apparently in part due to increase of cytosolic Ca2+ activity ([Ca2+]i), as they were significantly blunted by removal of extracellular Ca2+. An increase of [Ca2+]i triggers cell membrane scrambling by simulating an illdefined scramblase [26]. An increase of [Ca2+]i further triggers erythrocyte shrinkage by activation of Ca2+ sensitive K+ channels with subsequent K+ exit, cell membrane hyperpolarization, Cl- exit and thus cellular loss of KCl with water [25]. As an increase of [Ca2+]i triggers both, annnexin binding and cell shrinkage, the subpopulation of shrunken erythrocytes mainly includes erythrocytes undergoing eryptosis.
Removal of extraellular Ca2+ does not completely abrogate the effects of pentostatin on cell membrane scrambling and cell shrinkage. Thus, pentostatin may trigger cell membrane scrambling and cell shrinkage through some additional mechanism. Our observations did not reveal an effect of pentostatin on the abundance of ceramide, a well known stimulator of cell membrane scrambling not requiring increases of [Ca2+]i [26]. Our observations disclosed, however, that pentostatin exposure leads to ATP depletion, which is a powerful stimulator of eryptosis [26]. An effect of pentostatin on ATP has been shown previously [44, 45].
Consequences of stimulated eryptosis include anemia due to rapid clearance of eryptotic erythrocytes from circulating blood [26]. As eryptosis triggers disposal of defective erythrocytes prior to hemolysis [26], it prevents release of hemoglobin, which would otherwise be filtered in renal glomerula, precipitate in the acidic lumen of renal tubules and thus occlude nephrons [46]. Eryptosis may be particularly important in malaria [47]. The malaria pathogen Plasmodium imposes oxidative stress on the infected host erythrocyte, which leads to activation of several host cell ion channels including Ca2+-permeable erythrocyte cation channels [26, 48]. The subsequent Ca2+ entry triggers eryptosis with clearance of infected erythrocytes from circulating blood [47]. Sickle-cell trait, beta-thalassemia-trait, Hb-C and G6PD-deficiency erythrocytes are particularly sensitive to triggers of eryptosis and thus protect against a severe course of malaria by accelerating the removal of infected erythrocytes [26, 49-51]. Iron deficiency [52], and treatment with lead [52], chlorpromazine [53] or NO synthase inhibitors [53] similarly counteract parasitemia by accelerating eryptosis. It remains to be shown, whether pentostatin similarly fosters eryptosis in plasmodium infected erythrocytes.
Consequences of eryptosis may further include impairment of microcirculation [26]. Phosphatidylserine exposing erythrocytes may adhere to the vascular wall [54], trigger blood clotting and elicit thrombosis [55-57], events interering with microcirculation [27, 55, 58-61].
Pentostatin triggers eryptosis with cell shrinkage and cell membrane scrambling, an effect paralleled by and in part due to Ca2+ entry and energy depeletion.
The authors acknowledge the meticulous preparation of the manuscript by Tanja Loch. The study was supported by the Deutsche Forschungsgemeinschaft.
Dillman RO: Pentostatin (Nipent) in the treatment of chronic lymphocyte leukemia and hairy cell leukemia. Expert Rev Anticancer Ther 2004;4:27-36.
https://doi.org/10.1586/14737140.4.1.27Grever MR, Doan CA, Kraut EH: Pentostatin in the treatment of hairy-cell leukemia. Best Pract Res Clin Haematol 2003;16:91-99.
https://doi.org/10.1016/S1521-6926(03)00002-1Johnston JB: Mechanism of action of pentostatin and cladribine in hairy cell leukemia. Leuk Lymphoma 2011;52:43-45.
https://doi.org/10.3109/10428194.2011.570394Sauter C, Lamanna N, Weiss MA: Pentostatin in chronic lymphocytic leukemia. Expert Opin Drug Metab Toxicol 2008;4:1217-1222.
https://doi.org/10.1517/17425255.4.9.1217Spiers AS: Deoxycoformycin (pentostatin): clinical pharmacology, role in the chemotherapy of cancer, and use in other diseases. Haematologia (Budap) 1996;27:55-84.
Ho AD, Hensel M: Pentostatin and purine analogs for indolent lymphoid malignancies. Future Oncol 2006;2:169-183.
https://doi.org/10.2217/14796694.2.2.169Byrd JC, Grever MR, Waselenko JK, Willis CR, Park K, Goodrich A, Lucas MA, Shinn C, Flinn IW: Theophylline, pentostatin (Nipent), and chlorambucil: a dose-escalation study targeting intrinsic biologic resistance mechanisms in patients with relapsed lymphoproliferative disorders. Semin Oncol 2000;27:37-40.
Ho AD, Hensel M: Pentostatin for the treatment of indolent lymphoproliferative disorders. Semin Hematol 2006;43:S2-10.
https://doi.org/10.1053/j.seminhematol.2005.12.005Kurzrock R: Therapy of T cell lymphomas with pentostatin. Ann NY Acad Sci 2001;941:200-205.
https://doi.org/10.1111/j.1749-6632.2001.tb03724.xMargolis J, Grever MR: Pentostatin (Nipent): a review of potential toxicity and its management. Semin Oncol 2000;27:9-14.
Morabito F, Callea I, Console G, Stelitano C, Sculli G, Filangeri M, Oliva B, Musolino C, Iacopino P, Brugiatelli M: The in vitro cytotoxic effect of mitoxantrone in combination with fludarabine or pentostatin in B-cell chronic lymphocytic leukemia. Haematologica 1997;82:560-565.
Dearden CE, Else M, Catovsky D: Long-term results for pentostatin and cladribine treatment of hairy cell leukemia. Leuk Lymphoma 2011;52:21-24.
https://doi.org/10.3109/10428194.2011.565093Else M, Osuji N, Forconi F, Dearden C, Del Giudice I, Matutes E, Wotherspoon A, Lauria F, Catovsky D: The role of rituximab in combination with pentostatin or cladribine for the treatment of recurrent/refractory hairy cell leukemia. Cancer 2007;110:2240-2247.
https://doi.org/10.1002/cncr.23032Golomb HM, Dodge R, Mick R, Budman D, Hutchison R, Horning SJ, Schiffer CA: Pentostatin treatment for hairy cell leukemia patients who failed initial therapy with recombinant alpha-interferon: a report of CALGB study 8515. Leukemia 1994;8:2037-2040.
Grever MR: Pentostatin: impact on outcome in hairy cell leukemia. Hematol Oncol Clin North Am 2006;20:1099-1108.
https://doi.org/10.1016/j.hoc.2006.06.001Grever MR: Hairy cell leukemia: a successful model for experimental therapeutics--pentostatin and new ideas. Leuk Lymphoma 2011;52:25-28.
https://doi.org/10.3109/10428194.2011.577851Senatore FJ, Dasanu CA: Synchronous gastric and ampullary adenocarcinomas in a hairy cell leukemia patient treated with pentostatin eight years prior. J Oncol Pharm Pract 2016;22:543-547.
https://doi.org/10.1177/1078155215574140Lamanna N, Kay NE: Pentostatin treatment combinations in chronic lymphocytic leukemia. Clin Adv Hematol Oncol 2009;7:386-392.
Waselenko JK, Grever MR, Beer M, Lucas MA, Byrd JC: Pentostatin (Nipent) and chlorambucil with granulocyte-macrophage colony-stimulating factor support for patients with previously untreated, treated, and fludarabine-refractory B-cell chronic lymphocytic leukemia. Semin Oncol 2000;27:44-51.
Heald P: Future directions for pentostatin (Nipent) usage in dermatology. Semin Oncol 2000;27:3-8.
Bajwa R, Savelli S, Gross T: Pentostatin for treatment of refractory autoimmune lymphoproliferative syndrome. Pediatr Blood Cancer 2011;57:336-337.
https://doi.org/10.1002/pbc.23144Higman M, Vogelsang GB, Chen A: Pentostatin - pharmacology, immunology, and clinical effects in graft-versus-host disease. Expert Opin Pharmacother 2004;5:2605-2613.
https://doi.org/10.1517/14656566.5.12.2605Margolis J, Vogelsang G: An old drug for a new disease: pentostatin (Nipent) in acute graft-versus-host disease. Semin Oncol 2000;27:72-77.
Bolanos-Meade J, Jacobsohn DA, Margolis J, Ogden A, Wientjes MG, Byrd JC, Lucas DM, Anders V, Phelps M, Grever MR, Vogelsang GB: Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol 2005;23:2661-2668.
https://doi.org/10.1200/JCO.2005.06.130Lang PA, Kaiser S, Myssina S, Wieder T, Lang F, Huber SM: Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am J Physiol Cell Physiol 2003;285:C1553-C1560.
https://doi.org/10.1152/ajpcell.00186.2003Lang E, Qadri SM, Lang F: Killing me softly - suicidal erythrocyte death. Int J Biochem Cell Biol 2012;44:1236-1243.
https://doi.org/10.1016/j.biocel.2012.04.019Abed M, Towhid ST, Mia S, Pakladok T, Alesutan I, Borst O, Gawaz M, Gulbins E, Lang F: Sphingomyelinase-induced adhesion of eryptotic erythrocytes to endothelial cells. Am J Physiol Cell Physiol 2012;303:C991-999.
https://doi.org/10.1152/ajpcell.00239.2012Lau IP, Chen H, Wang J, Ong HC, Leung KC, Ho HP, Kong SK: In vitro effect of CTAB- and PEG-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology 2012;6:847-856.
https://doi.org/10.3109/17435390.2011.625132Maellaro E, Leoncini S, Moretti D, Del Bello B, Tanganelli I, De Felice C, Ciccoli L: Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol 2013;50:489-495.
https://doi.org/10.1007/s00592-011-0274-0Lathia C, Fleming GF, Meyer M, Ratain MJ, Whitfield L: Pentostatin pharmacokinetics and dosing recommendations in patients with mild renal impairment. Cancer Chemother Pharmacol 2002;50:121-126.
https://doi.org/10.1007/s00280-002-0468-9Poi MJ, Hofmeister CC, Johnston JS, Edwards RB, Jansak BS, Lucas DM, Farag SS, Dalton JT, Devine SM, Grever MR, Phelps MA: Standard pentostatin dose reductions in renal insufficiency are not adequate: selected patients with steroid-refractory acute graft-versus-host disease. Clin Pharmacokinet 2013;52:705-712.
https://doi.org/10.1007/s40262-013-0064-7Abed M, Feger M, Alzoubi K, Pakladok T, Frauenfeld L, Geiger C, Towhid ST, Lang F: Sensitization of erythrocytes to suicidal erythrocyte death following water deprivation. Kidney Blood Press Res 2013;37:567-578.
https://doi.org/10.1159/000355737Voelkl J, Alzoubi K, Mamar AK, Ahmed MS, Abed M, Lang F: Stimulation of suicidal erythrocyte death by increased extracellular phosphate concentrations. Kidney Blood Press Res 2013;38:42-51.
https://doi.org/10.1159/000355752Abed M, Artunc F, Alzoubi K, Honisch S, Baumann D, Foller M, Lang F: Suicidal erythrocyte death in end-stage renal disease. J Mol Med (Berl) 2014;92:871-879.
https://doi.org/10.1007/s00109-014-1151-4Ahmed MS, Langer H, Abed M, Voelkl J, Lang F: The uremic toxin acrolein promotes suicidal erythrocyte death. Kidney Blood Press Res 2013;37:158-167.
https://doi.org/10.1159/000350141Calderon-Salinas JV, Munoz-Reyes EG, Guerrero-Romero JF, Rodriguez-Moran M, Bracho-Riquelme RL, Carrera-Gracia MA, Quintanar-Escorza MA: Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol Cell Biochem 2011;357:171-179.
https://doi.org/10.1007/s11010-011-0887-1Polak-Jonkisz D, Purzyc L: Ca(2+) influx versus efflux during eryptosis in uremic erythrocytes. Blood Purif 2012;34:209-210; author reply 210.
https://doi.org/10.1159/000341627Lang PA, Beringer O, Nicolay JP, Amon O, Kempe DS, Hermle T, Attanasio P, Akel A, Schafer R, Friedrich B, Risler T, Baur M, Olbricht CJ, Zimmerhackl LB, Zipfel PF, Wieder T, Lang F: Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J Mol Med (Berl) 2006;84:378-388.
https://doi.org/10.1007/s00109-006-0058-0Nicolay JP, Schneider J, Niemoeller OM, Artunc F, Portero-Otin M, Haik G, Jr., Thornalley PJ, Schleicher E, Wieder T, Lang F: Stimulation of suicidal erythrocyte death by methylglyoxal. Cell Physiol Biochem 2006;18:223-232.
https://doi.org/10.1159/000097669Lang E, Gatidis S, Freise NF, Bock H, Kubitz R, Lauermann C, Orth HM, Klindt C, Schuier M, Keitel V, Reich M, Liu G, Schmidt S, Xu HC, Qadri SM, Herebian D, Pandyra AA, Mayatepek E, Gulbins E, Lang F, et al.: Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 2015;61:275-284.
https://doi.org/10.1002/hep.27338Qadri SM, Mahmud H, Lang E, Gu S, Bobbala D, Zelenak C, Jilani K, Siegfried A, Foller M, Lang F: Enhanced suicidal erythrocyte death in mice carrying a loss-of-function mutation of the adenomatous polyposis coli gene. J Cell Mol Med 2012;16:1085-1093.
https://doi.org/10.1111/j.1582-4934.2011.01387.xKempe DS, Akel A, Lang PA, Hermle T, Biswas R, Muresanu J, Friedrich B, Dreischer P, Wolz C, Schumacher U, Peschel A, Gotz F, Doring G, Wieder T, Gulbins E, Lang F: Suicidal erythrocyte death in sepsis. J Mol Med (Berl) 2007;85:273-281.
https://doi.org/10.1007/s00109-006-0123-8Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F: Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 2007;13:164-170.
https://doi.org/10.1038/nm1539Siaw MF, Mitchell BS, Koller CA, Coleman MS, Hutton JJ: ATP depletion as a consequence of adenosine deaminase inhibition in man. Proc Natl Acad Sci U S A 1980;77:6157-6161.
https://doi.org/10.1073/pnas.77.10.6157Buc HA, Thuillier L, Hamet M, Garreau F, Moncion A, Perignon JL: Energy metabolism in adenosine deaminase-inhibited human erythrocytes. Clin Chim Acta 1986;156:61-69.
https://doi.org/10.1016/0009-8981(86)90179-8Harrison HE, Bunting H, Ordway NK, Albrink WS: The Pathogenesis of the Renal Injury Produced in the Dog by Hemoglobin or Methemoglobin. J Exp Med 1947;86:339-356.
https://doi.org/10.1084/jem.86.4.339Foller M, Bobbala D, Koka S, Huber SM, Gulbins E, Lang F: Suicide for survival--death of infected erythrocytes as a host mechanism to survive malaria. Cell Physiol Biochem 2009;24:133-140.
https://doi.org/10.1159/000233238Kirk K: Membrane transport in the malaria-infected erythrocyte. Physiol Rev 2001;81:495-537.
https://doi.org/10.1152/physrev.2001.81.2.495Ayi K, Giribaldi G, Skorokhod A, Schwarzer E, Prendergast PT, Arese P: 16alpha-bromoepiandrosterone, an antimalarial analogue of the hormone dehydroepiandrosterone, enhances phagocytosis of ring stage parasitized erythrocytes: a novel mechanism for antimalarial activity. Antimicrob Agents Chemother 2002;46:3180-3184.
https://doi.org/10.1128/AAC.46.10.3180-3184.2002Ayi K, Turrini F, Piga A, Arese P: Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood 2004;104:3364-3371.
https://doi.org/10.1182/blood-2003-11-3820Cappadoro M, Giribaldi G, O'Brien E, Turrini F, Mannu F, Ulliers D, Simula G, Luzzatto L, Arese P: Early phagocytosis of glucose-6-phosphate dehydrogenase (G6PD)-deficient erythrocytes parasitized by Plasmodium falciparum may explain malaria protection in G6PD deficiency. Blood 1998;92:2527-2534.
https://doi.org/10.1182/blood.V92.7.2527.2527_2527_2534Koka S, Huber SM, Boini KM, Lang C, Foller M, Lang F: Lead decreases parasitemia and enhances survival of Plasmodium berghei-infected mice. Biochem Biophys Res Commun 2007;363:484-489.
https://doi.org/10.1016/j.bbrc.2007.08.173Koka S, Lang C, Niemoeller OM, Boini KM, Nicolay JP, Huber SM, Lang F: Influence of NO synthase inhibitor L-NAME on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem 2008;21:481-488.
https://doi.org/10.1159/000129641Borst O, Abed M, Alesutan I, Towhid ST, Qadri SM, Foller M, Gawaz M, Lang F: Dynamic adhesion of eryptotic erythrocytes to endothelial cells via CXCL16/SR-PSOX. Am J Physiol Cell Physiol 2012;302:C644-C651.
https://doi.org/10.1152/ajpcell.00340.2011Andrews DA, Low PS: Role of red blood cells in thrombosis. Curr Opin Hematol 1999;6:76-82.
https://doi.org/10.1097/00062752-199903000-00004Chung SM, Bae ON, Lim KM, Noh JY, Lee MY, Jung YS, Chung JH: Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler Thromb Vasc Biol 2007;27:414-421.
https://doi.org/10.1161/01.ATV.0000252898.48084.6aZwaal RF, Comfurius P, Bevers EM: Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci 2005;62:971-988.
https://doi.org/10.1007/s00018-005-4527-3Closse C, Dachary-Prigent J, Boisseau MR: Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br J Haematol 1999;107:300-302.
https://doi.org/10.1046/j.1365-2141.1999.01718.xGallagher PG, Chang SH, Rettig MP, Neely JE, Hillery CA, Smith BD, Low PS: Altered erythrocyte endothelial adherence and membrane phospholipid asymmetry in hereditary hydrocytosis. Blood 2003;101:4625-4627.
https://doi.org/10.1182/blood-2001-12-0329Pandolfi A, Di Pietro N, Sirolli V, Giardinelli A, Di Silvestre S, Amoroso L, Di Tomo P, Capani F, Consoli A, Bonomini M: Mechanisms of uremic erythrocyte-induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol 2007;213:699-709.
https://doi.org/10.1002/jcp.21138Wood BL, Gibson DF, Tait JF: Increased erythrocyte phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood 1996;88:1873-1880.
https://doi.org/10.1182/blood.V88.5.1873.bloodjournal8851873