

Original Article - DOI:10.33594/000000627
Accepted 11 April 2023 - Published online 14 May 2023
1Cell Biophysics Group, Department of Pharmacology & Toxicology, Boonshoft School of Medicine, Wright State University, Dayton, OH, USA;
2Biomedical Sciences Ph.D. Program, Wright State University, Dayton, OH, USA
Background/Aims: Apelin and its signaling through the G-protein coupled receptor (APJ, gene symbol APLNR) regulate cardiovascular function via two mechanisms: 1) By promoting nitric oxide (NO)-mediated vasodilation, impaired by oxidized low-density lipoproteins (oxLDL); and 2) By inducing cell proliferation via the phosphatidylinositol-3-kinase (PI3K)/protein kinase B/Akt pathway (PI3K/Akt) and mitogen activated protein kinase (MAPK) pathways. The potassium chloride cotransporter (KCC1,3,4; SLC12A4,6,7) controls cell volume, and regulates cardiovascular function through proliferation, migration, and blood pressure control. Importantly, KCC regulatory mechanisms and apelin/APJ signaling pathways overlap placing KCC as a potential target for apelin/APJ to elicit its cardioprotective effects. Therefore, apelin’s action on KCC activity was examined in contractile and synthetic rat aortic vascular smooth muscle cells (VSMCs). Methods: KCC activity was measured by atomic absorption spectrophotometry in chloride (Cl-) and Cl--free medium with sulfamate (Sf-) as Cl- replacement, and with rubidium (Rb+) as a potassium (K+) congener. The calculated difference between Rb+ transport in the presence of chloride (Cl-) and sulfamate (Sf-) is the Cl--dependent Rb+ influx (i.e., K-Cl cotransport activity). Apelin-13 (1 µM) was added either during flux (acute effect) and/or in the growth media (chronic effect) based on the experimental goals. KCC activity was characterized with respect to the VSMC phenotypes, in the presence or absence of apelin and corresponding inhibitors of the signaling pathways, oxLDL, and as a function of various physiological factors described below. Results: The APJ receptor was expressed in both contractile and synthetic VSMC phenotypes, the former also possessing the soluble guanylyl cyclase-coupled protein kinase G (PKG) receptor, critical for NO-mediated signaling. In general, KCC activity was higher in synthetic vs. contractile VSMCs, consistent with enhanced migration and proliferation in the former. In addition, apelin-mediated activation of KCC was modulated by extracellular sodium [Na+]o, osmolality, length of apelin treatment (acute or chronic) and VSMC phenotype (contractile vs synthetic). Based on selective inhibitors, apelin activated KCC through the (NO)/soluble guanylate cyclase (sGC)/protein kinase G (PKG) (NO/sGC/PKG)-, PI3K/Akt- and MAPK-dependent pathway(s). Furthermore, apelin rescued the inhibition of KCC by oxLDL. Altogether, results suggest apelin/APJ as an important modulator of KCC activity to sustain cell volume regulation and cardiovascular function. More recently, the apelinergic system has been proposed as a novel target for the treatment of Corona virus disease 2019 (COVID-19) and, given the significant overlap between the regulatory mechanisms of this system and KCC, and their role in cardiovascular disease (CVD), this study opens new avenues to identify potential targets for diverse implementation strategies. Conclusion: A better understanding of apelin effects on KCC will help design a novel therapeutic approach to treat atherosclerosis-linked cardiovascular diseases, including COVID-19-associated mortality.
Among several risk factors that lead to cardiovascular disease (CVD), atherosclerosis plays a pivotal role ([1], and references therein) due to various determinants such as: 1) Loss of contractility of vascular smooth muscle cells (VSMCs) and their irreversible remodeling [2]; 2) Significant changes in size, density, and chemical properties of circulating low-density lipoproteins (LDL); 3) Oxidative stress, and 4) Endothelial dysfunction resulting in luminal narrowing and concomitant inflammatory events that trigger sub-endothelial accumulation of oxidized LDL (oxLDL), macrophage infiltration and, ultimately, lesion formation (foam cell appearance of macrophages and atherosclerotic plaques) ([1], and references therein; [3], and references therein).
Studies of VSMCs proliferation and migration are key to understanding atherosclerotic plaque repair. The coordinated activities of the Na+-K+-2Cl- and K+-Cl- cotransporters (NKCCs and KCCs, respectively) are central to cell volume control and thereby cell proliferation and migration. Cell volume reduction results in a corrective influx of Na+, K+, Cl- and water via NKCC, whereas cell volume increase promotes the efflux of K+, Cl- and water by stimulating the activity of KCC ([4], and references therein; [5], and references therein; [6]).The roles of KCC and NKCC in cell proliferation and migration are also an active area of investigation in cancer and involve at least two mechanisms: 1) Regulation of structural components, such as assembly of actin filaments, focal cell adhesions, lamellipodia protrusion, and changes in shape and migration that ultimately result in the transition from normal to cancerous cells with enhanced invasiveness, and metastatic properties; and 2) Regulation of ion transport by changes in cell volume to avoid/reverse carcinogenesis or to implement strategies to stop cancer progression, removal, or reversal ([7], and references therein; [8-10]). Mechanisms similar to those in cancer, i.e., through regulation of cell structure and volume via signaling pathways, may be at play in VSMCs during atherosclerotic plaque formation, thus by applying counteractive measures, these may lead to its reversal.
Recently, the signaling pathway of apelin and its G-protein coupled receptor (APJ), were recognized as a potent regulator of cardiovascular function ([11], and references therein; [10, 12, 13]; [14], and references therein). Apelin knockout mice models display impaired cardiac contractility and development of CVD ([11], and references therein). The apelin/APJ system plays a central role in cell function creating a superb therapeutic target for the treatment of CVD ([14], and references therein; [15]). Apelin, generated in vivo as a 77 amino acid precursor (pre-proapelin), is cleaved into functional shorter peptides (Apelin-13,-16,-17,-19 and -36). Apelin-13 was chosen in the present study due to its highest activity and concentration in the circulatory system ([14], and references therein; [15]; [16], and references therein). Besides, among apelin’s cardioprotective effects, there is evidence for strong anti-hypertensive and anti-atherogenicproperties, both associated with several intracellular signaling cascades such as a) the nitric oxide (NO)/soluble guanylate cyclase (sGC)/protein kinase G (PKG) pathway (NO/sGC/PKG) pathway, b) phosphatidylinositol-3-kinase (PI3K)/protein kinase B/Akt pathway (PI3K/Akt), and c) the mitogen activated protein kinase (MAPK) signaling network Ras/Raf/ERK (MAPK/ERK1/2) ([11], and references therein; [13, 15, 17, 18]; [14], and references therein; [16], and references therein).
Analogous and overlapping regulatory networks converge to coordinate apelin-mediated effects that could readjust KCC activity through phosphorylation/dephosphorylation events. For example, high levels of oxLDL uptake by the fatty acid translocase CD36 (atherosclerosis hallmark) result in pronounced upregulation of KCC1 [19]. At the functional and molecular levels, KCC has been shown to be modulated by the NO, PI3K/Akt and MAPK/ERK1/2 signaling networks ([4], and references therein; [5], and references therein; [20, 21]), pathways through which apelin also elicits its cardioprotective effects. Furthermore, KCC isoforms are important not only for controlling cell migration and volume, but also for sustaining blood pressure levels, as shown in mice KCC3 knockout models which develop severe hypertension and hence abnormal cardiovascular function ([4], and references therein) likely due to the predominant KCC isoforms expressed in VSMCs, KCC1, KCC3 and KCC4 ([4], and references therein; [20]).
KCC and NKCC work in a yin-yang manner to regulate the cellular osmotic balance. Likewise, apelin and the antidiuretic hormone vasopressin reciprocally regulate osmotic balance for body fluid homeostasis ([11], and references therein; [22, 23]). In humans and rodents, the short-term and long-term administration of vasopressin increases NKCC expression possibly due to its phosphorylation [24]. Similarly, vasopressin, hyperosmolality, and dehydration increase NKCC mRNA and protein expression [25]. In contrast to vasopressin, osmotic challenge resulting from hypoosmolality and water loading increases plasma apelin concentration [22]. Thus, changes in osmolality induced by the coordinated action of apelin and vasopressin may affect the activity of KCC and NKCC reciprocally. Hence, KCC regulation turns out to be important for maintenance of cardiovascular function, such as vasodilation, and the amelioration of CVD.
The present study determined the effect of apelin-13 on KCC activity using primary cultures of contractile and synthetic VSMCs. Although KCC is known to be a Na+-independent transporter in most cells and tissues, including our own studies ([4], and references therein; [5], and references therein; [21]), in the present investigation KCC was measured as a Cl--dependent transporter. Thus, under certain conditions, a residual presence of NKCC could become a confounding factor in the assessment of KCC activity. Therefore, several factors such as [Na+]o, osmolality, length of apelin treatment (acute or chronic), and VSMC phenotype (contractile vs synthetic) were assessed to optimize KCC’s activity under the various conditions tested.
Remarkably, apart from its role in CVD, the apelinergic system [13] has been a novel therapeutic target in the treatment of severe acute respiratory syndrome (SARS) caused by coronaviruses (SARS-CoV-2), the causative agents of COVID-19, that resulted in a global pandemic in 2020 [26, 27]. Recently, Apelin and the APJ receptor have been proposed as important therapeutic targets for the treatment of COVID-19 infection by inhibiting the virus entry into the cells via the ACE2 receptor [28]. The present work could be of further importance not only to treat CVD but also because by activating the apelin signaling mechanism and stimulating KCC, could help COVID-19 patients to further ameliorate their conditions, as activation of KCC favors vasodilation and potentially decrease the chances of viral invasion, thereby alleviating cardiovascular and lung injury in COVID patients. Therefore, upregulating apelin, its receptor APJ, inhibiting viral entry to the cells, and, activating the new potential target, KCC, could offer at least four therapeutic targets to counteract the effects of COVID, and ultimately attenuate the COVID-19 pandemic.
Apelin-13, the most abundant isoform in circulation in vivo, has been used in all the experiments of the present study.
Chemicals, reagents, and antibodies
Rubidium chloride (RbCl, 99.8 % purity metal basis) and amidosulfonic acid (99.99 % purity, metal basis) were acquired from Alfa Aesar (Ward Hill, MA). Potassium chloride (KCl), sodium hydroxide (NaOH), magnesium chloride (MgCl2), 2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid (HEPES), Tris, D-glucose, sucrose, calcium chloride (CaCl2), calcium gluconate, N-methyl D-glucamine (NMDG), Minimum Essential Medium (MEM) Alpha Medium, low glucose Dulbecco’s Modified Eagle’s culture medium (DMEM), fetal bovine serum (FBS), 0.25 % trypsin, and 70 % perchloric acid (PCA) were purchased from Thermo Fisher Scientific (Waltham, MA). Cesium chloride (CsCl), penicillin (100 units/ml) and streptomycin (100 µg/ml) were acquired from Life Technologies (Carlsbad, CA), now Thermo Fisher Sci. (Grand Island, NY). Magnesium gluconate, 3-[N-morpholino] propane sulfonic acid (MOPS), and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Ouabain octahydrate was purchased from EMD Millipore (Billerica, MA). Bumetanide was acquired from MP Biomedicals (Solon, OH). Bicinchoninic acid (BCA) protein kit, mammalian protein extraction reagent (M-Per), radio-immunoprecipitation assay (RIPA) buffer (catalog # 898900), Halt protease inhibitor and enhanced chemiluminescence (ECL) substrate were from Pierce (Rockford, IL).
Rabbit (rb) polyclonal anti-apelin receptor (APJ) antibody [29] was purchased from EMD Millipore (Billerica, MA). Polyclonal rb anti-PKG antibody was purchased from BioVision (San Francisco, CA). Secondary antibodies CY3 donkey anti-rb and FITC donkey anti-rb obtained from Jackson ImmunoResearch Laboratory (West Grove, PA).
Apelin-13 (catalog # 833) was acquired from Anaspec (Fremont, CA). Inhibitors of the PKG, PI3K/Akt and MAPK signaling pathways (KT5823 (catalog # 420321), LY294002 (catalog # 19-142) and PD98059 (catalog # 513000), respectively) were purchased from Calbiochem (Billerica, MA). oxLDL (catalog # STA-214) was purchased from Cell BioLabs (San Diego, CA). Collagenase type II (catalog # 171049-019) was purchased from GIBCO BRL (San Francisco, CA). The Bradford protein assay (catalog # 5000002) was purchased from Bio-Rad (Hercules, CA).
Primary cultures of rat aortic VSMCs
During the determination of KCC activity, primary VSMC preparations were isolated from control (untreated) rats obtained at the Wright State University Laboratory Animal Resources as previously described ([4], and references therein; [5], and references therein). During the course of these studies, the apelin effect was studied either in serum-starved or serum-fed VSMCs, depending on the experimental conditions, and the requirements for activators or inhibitors used according to the manufacturer’s instructions. Primary cultures of rat VSMCs were harvested and maintained according to previously described protocols ([4], and references therein; [5], and references therein). Briefly, cells were cultured in DMEM growth media containing 10 % FBS supplemented with penicillin and streptomycin (100 units/mL). Cells were grown in T-75 flasks in a fully humidified incubator equipped with 95 % O2/5 % CO2 at 37 °C. Upon confluency, cells were subcultured into 12-well plates or 100 cm Petri dishes according to the experimental goal. Confluent VSMCs at passages 1–4 were used as contractile VSMCs whereas passages 5-40 served as synthetic VSMCs based on previous phenotype characterization reports [30-32].
Balanced salt solutions for Rb+ transport studies
The balanced salt solution (BSS-NaCl or NMDGCl where appropriate) was composed of 20 mM HEPES-Tris buffer (pH 7.4 at 37 °C) containing (in mM): NaCl (130), KCl (5), CaCl2 (2), MgCl2 (1), glucose (10). The preincubation solution had 0.1 % BSA in BSS. The Na-free media contained NMDG. The Cl--free media had sulfamate (Sf-) in K+, Rb+, and Na+ salts, and gluconate in Mg+2 and Ca+2 salts. A wash buffer to terminate Rb+ uptake was composed of 10 mM MOPS-TRIS and 108 mM MgCl2 at pH 7.4 (300 mOsM). Stock salt solutions were prepared using deionized water. Osmolality was changed by increasing the ionic strength of the solution with variable amounts of NaCl, NaSf, NMDGCl or NMDGSf salts while keeping the other ions constant. Osmolality (in mOsmol/Kg H2O) of the hypotonic, isotonic, and hypertonic solutions were 120, 300 and 450, respectively. Ouabain (2 mM) and bumetanide (2-30 µM) were used to inhibit Rb+/K+ fluxes through the endogenous Na+/K+ pump and Na+-K+-2Cl- cotransporter, respectively. The effect of bumetanide on KCC in our primary VSMCs model was tested in dose-response curves as a function of the bumetanide concentration and at different osmolalities to ensure no uninhibited NKCC remained at the osmolalities tested (Results not shown). Based on these studies, bumetanide was used at the appropriate concentrations needed. The osmolalities of all prepared solutions were measured and confirmed with an Advanced Micro-Osmometer (Norwood, MA).
Rb+ transport studiesK-Cl cotransport activity was measured by previously established protocols in VSMCs ([4], and references therein; [5], and references therein). VSMCs cultured on 6-12-well plates to sub-confluency were serum-deprived for 24 h, unless otherwise specified. Cells were rinsed three times with BSS containing Na or NMDG where appropriate and then equilibrated for 10 min in preincubation media containing 0.1 % BSA in BSS Na or NMDG solution. Next, cells were incubated at various times (0-40 min) in flux media containing 10 mM RbCl to serve as a K+ congener, and Na or NMDG salts, as described above. Ouabain and bumetanide were added to preincubation and flux media, unless otherwise specified. Test drugs, such as inhibitors of signaling pathways and oxLDL, were added either during preincubation, flux or both, or for up to 24 h in culture media with or without serum, per the manufacturer’s instructions. For acute apelin-13 treatment, the drugs were present either during preincubation and flux or flux alone, whereas in chronic treatments it was added in the culture media and the incubation lasted up to 24 h. Further details are reported in the figure legends. Flux was terminated by washing cells with ice cold washing solution. Intracellular Rb+ content was measured by incubating cells with PCA and CsCl and the amount of Rb+ taken up by the cells was analyzed using a Perkin Elmer 5000 atomic absorption spectrophotometer as described elsewhere ([4], and references therein; [5], and references therein). Cells were digested in NaOH, and the protein concentration was determined using the BCA protein assay. The calculated difference between Rb+ transport in the presence of Cl- and Sf - is defined as Cl--dependent Rb+ influx (i.e., K-Cl cotransport activity). Apelin-13 (1 µM) was added either during flux and/or in the growth media based on the experimental goals and design specified in the Results section.
Immunofluorescence
To examine the localization pattern of the APJ receptor and PKG, VSMCs were cultured in 8-well chamber slides at 8 ⨯ 104 cells/well seeding density according to the protocol described elsewhere [5, 33] with modification in the saponin concentration (0.1 % instead of 0.01 %) and in the seeding density, as indicated above. Cells were washed with phosphate buffered saline (PBS), fixed and permeabilized with 4 % paraformaldehyde and 0.1 % saponin for 30 min at 4 °C. After fixation, cells were rinsed with PBS and blocked with 5 % normal goat serum (NGS) in PBS for 1 h at 4 °C. Rabbit (rb) polyclonal antibodies against the APJ receptor [1:100] [29] and PKG [1:100] were used for immunostaining. Cells were incubated with primary antibodies plus 5 % NGS in PBS overnight at 4 °C. The following day, the cells were rinsed with PBS and then exposed to secondary anti-rb fluorescein-conjugated isothiocyanate (FITC) antibody [1:100] and anti-rb Cy3 [1:200] in 5 % NGS for 1 h. Cells were co-stained with 4’,6-diamidino-2-phenylindole (300 nM DAPI) for cell nuclei visualization. Slides were visualized using an inverted Nikon E400 fluorescent microscope under 100 X (oil) objectives and overlaid using GNU image manipulation program (GIMP) software.
Statistical analysis
Graphs were generated using Origin 7.0 (Origin Labs, Northampton MA) and STATISTIX 7 software (Analytical software, Tallahassee FL). Most results shown are product of at least two independent experiments (N) with multiple determinations per condition (n). Data are reported as mean ± SD or SEM. Paired, unpaired t-test, or one way ANOVA followed by Tukey’s post-hoc test analysis, as appropriate, was performed using Statistix 7 or GraphPad Prism 5 software (La Jolla, CA). A p < 0.05 was considered statistically significant.
Apelin Receptor Expression in VSMCsExpression of the apelin’s APJ receptor was assessed by immunocytochemistry to validate the VSMCs model used in this study. Fig. 1 shows APJ’s presence with differences in its subcellular localization among VSMCs phenotypes. In synthetic VSMCs (passage number 17), the majority of APJ expression was homogeneously distributed in the cytoplasm (Fig. 1A), whereas a more localized perinuclear staining occurred in contractile cells (passage number 2) (Fig. 1C). Control VSMCs with only secondary antibody stained negative (Control in Fig. 1 B and D).
Fig. 1E confirms presence of PKG in contractile VSMCs by immunolabeling of the enzyme. Secondary nonspecific antibody immuno-reactivity in VSMCs was ruled out (Fig. 1F). Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) (Fig. 1A-F). Based on previous studies indicating lack of PKG in synthetic VSMCs ([4], and references therein; [5], and references therein; [32]; [34], and references therein) only contractile VSMCs were tested.
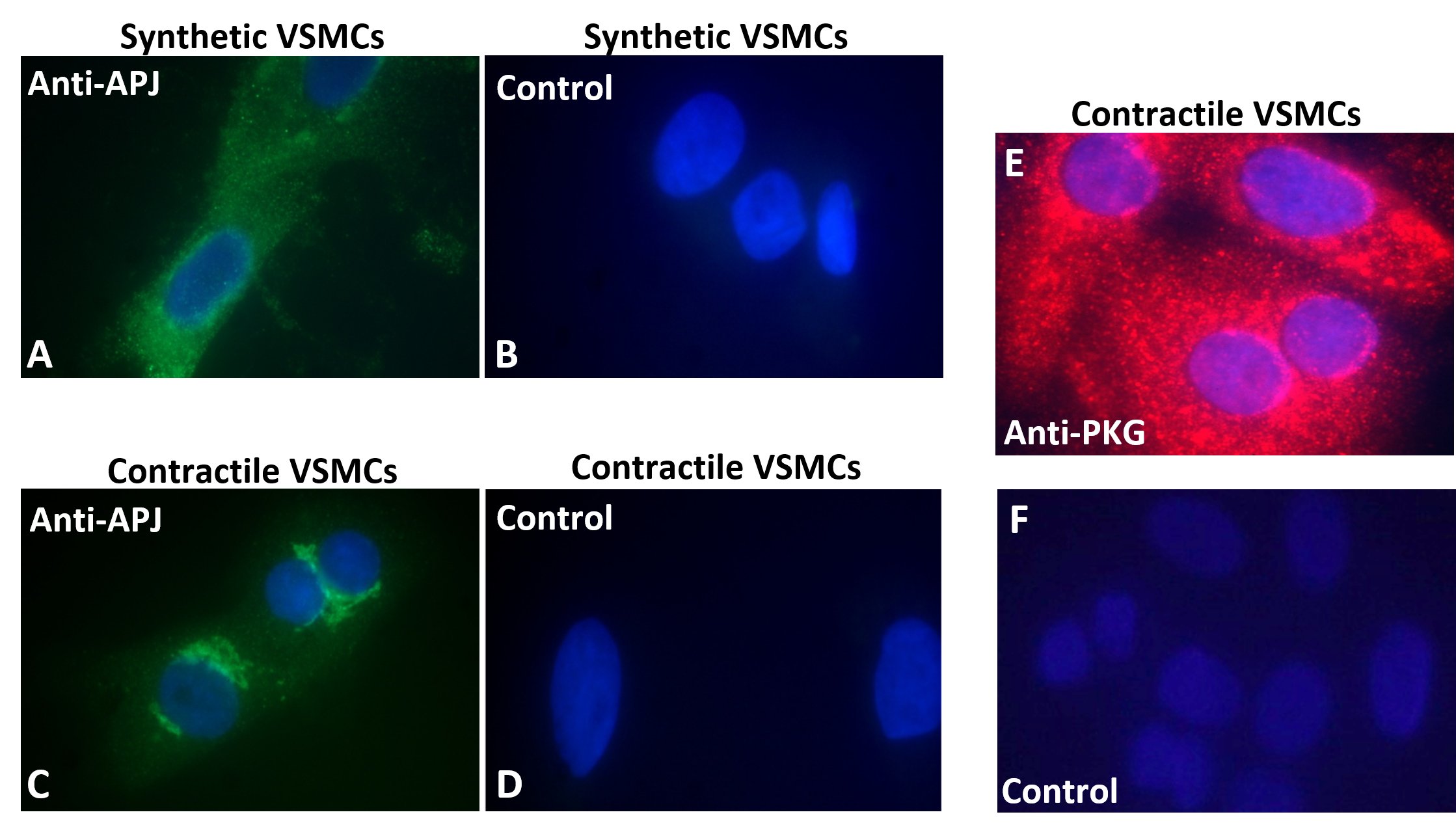
Figure 1. Immunocytochemistry. Immuno-staining of (A & B), APJ in synthetic (passage 17), and (C & D) in contractile (passage 2) VSMCs. In both cases (left panels, A & C), VSMCs were fixed, permeabilized, and incubated with anti-APJ antibody (1:100), followed by (FITC)-conjugated secondary antibody against IgG (1:100). Controls (right panels, B & D): 3% normal goat serum (NGS) and (FITC)-conjugated secondary antibody (1:100). (E & F) Immuno-staining of PKG in contractile VSMCs (passage 3). Cells were fixed, permeabilized and incubated with anti-PKG 1 antibody (1:100) followed by Cy3-conjugated secondary antibody (1:200) against IgG (upper panel E). Controls: 3 % NGS and Cy3-conjugated secondary antibody (1:200) (lower panel F). DAPI (300 nM) was used for nuclear staining.
KCC versus NKCCCation chloride cotransporters (CCCs), like KCC and NKCC, modulate the intracellular Cl- concentration and therefore cell volume through coordinated activities known as Yin-Yang effect. This required the present VSMCs model to behave in such a way when exposed to different osmolalities, i.e., hypotonic, isotonic, and hypertonic conditions. Fig. 2 confirms the coordinated activity of KCC and NKCC. As shown in Fig. 2A, hypotonicity and hypertonicity activated and inhibited KCC, respectively, whereas opposite regulation (inhibition and activation) of NKCC was observed (Fig. 2B). These results highlight the coordinate activity demonstrated in other cell types [35], in the primary cultures of synthetic vascular smooth muscle cells used in this study. This is important in light of the osmosensing behavior of apelin as described in healthy adults [22].
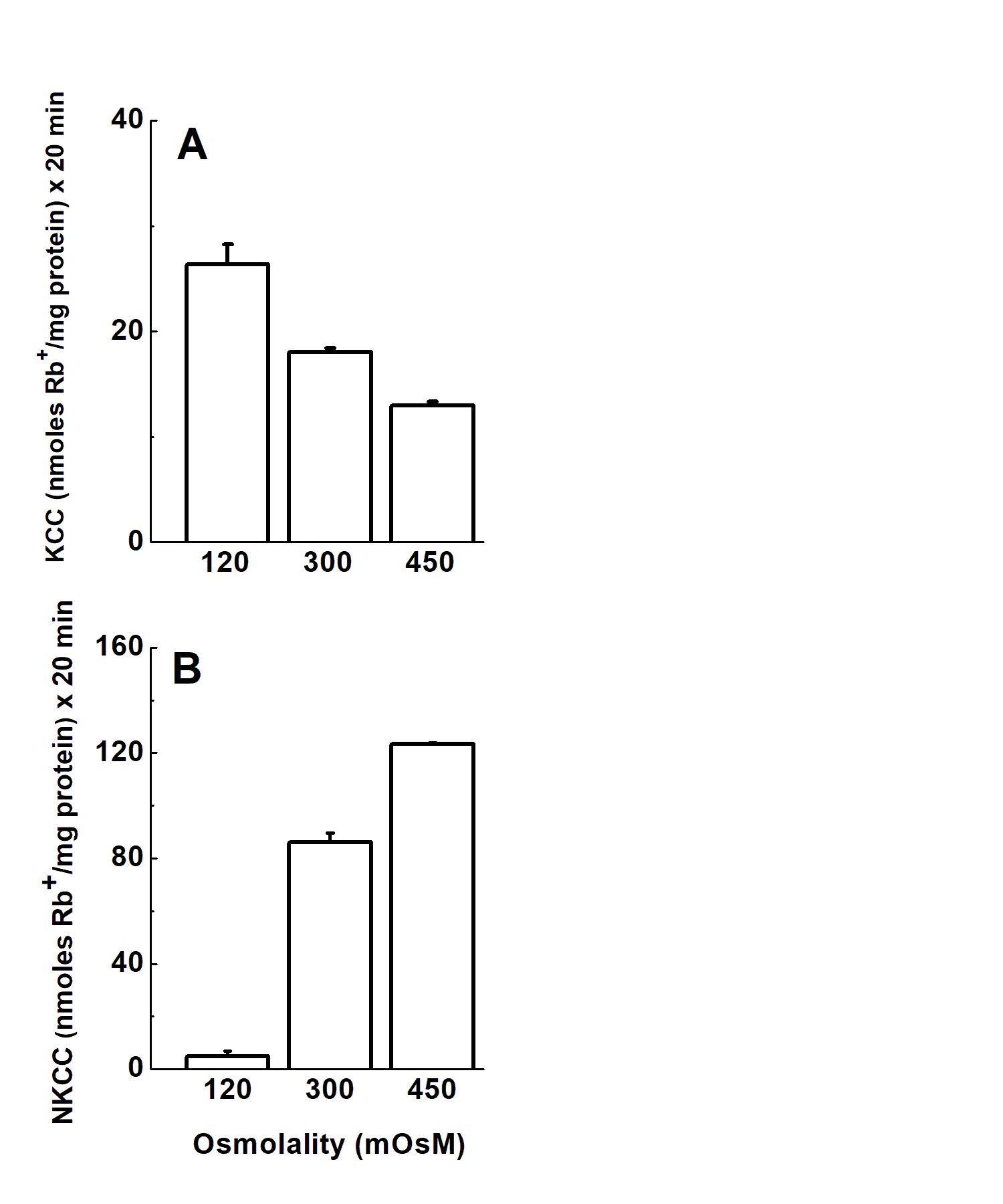
Figure 2. NKCC and KCC activation tightly and reciprocally regulated (Yin-Yang mechanism). To prove the Yin-Yang canonical mechanism between NKCC and KCC as a function of different tonicities, NaCl and NaSf were used to vary external tonicity. VSMCs (synthetic, passage 10) were cultured as described in Materials and Methods and Rb+ influx was measured in the presence of external Na+. Ouabain (2 mM) and bumetanide (30 µM) were used to inhibit the Na+/K+ pump and Na+-K+-2Cl+ cotransporter, respectively. The increase in bumetanide concentration was the result of dose-response studies of the inhibitor as a function of osmolality. Ouabain was used during flux alone and bumetanide was used during preincubation and flux. Data are mean ± standard error from three independent experiments each with n = 3 individual determinations per condition.
Variables Affecting the KCC Response to ApelinKCC activity was determined in the presence and absence of [Na+]o and at different osmolalities to ensure that it behaved as a Na+-independent transporter for the regulation of KCC by apelin. Fig. 3 shows the KCC activity as a function of osmolality in the absence (Panel A) and presence of external sodium ([Na+]o) (Panel B). In [Na+]o-free media, the familiar increase and decrease in KCC activity by hypo- and hyper-tonicity was absent, whereas in Panel B it was present. These data mean that [Na+]o was required for KCC activation and inhibition under hypotonicity and hypertonicity, respectively (Fig. 3B vs 3A).
In light of the KCC’s response to extracellular ionic content and osmolality, the effect of [Na+]o-free media on the apelin-mediated KCC response was investigated in both contractile and synthetic VSMCs, and as a function of increasing osmolality.
Fig. 4 shows the effect of [Na+]o-free media on the apelin effect as a function of increasing osmolality in contractile (Panel A) and synthetic (Panel B) VSMCs. Surprisingly, in the absence of [Na+]o there was no effect of apelin on KCC activity, irrespective of cell phenotype (synthetic or contractile) and extracellular osmolality. In addition, hypotonic incubation (which activates KCC) did not change Rb+ influx under this condition. As shown in Fig. 4, hypertonicity failed to change KCC activity in contractile controls and decreased it only by 10-20 % in synthetic cells as compared to isotonicity, whereas apelin treatment had no effect on Rb+ influx. These data clearly indicate a [Na+]o-dependency of KCC activity, which has not been shown in several cell types and certainly not in VSMCs before.
The apelin-mediated action on KCC and the [Na+]o effect on contractile and synthetic VSMCs was further tested at different osmolalities. As shown in Fig. 5A, apelin treatment in the presence of [Na+]o increased KCC in contractile VSMCs at all osmolalities tested, although the extent of activation was considerably less when compared to synthetic VSMCs in hypotonic and hypertonic conditions (Fig. 5B). It appears that the presence of [Na+]o allowed apelin to potentiate KCC under hypotonicity and hypertonicity in synthetic VSMCs. However, under isotonic conditions (300 mOsM), apelin treatment did not change baseline Rb+ influx.
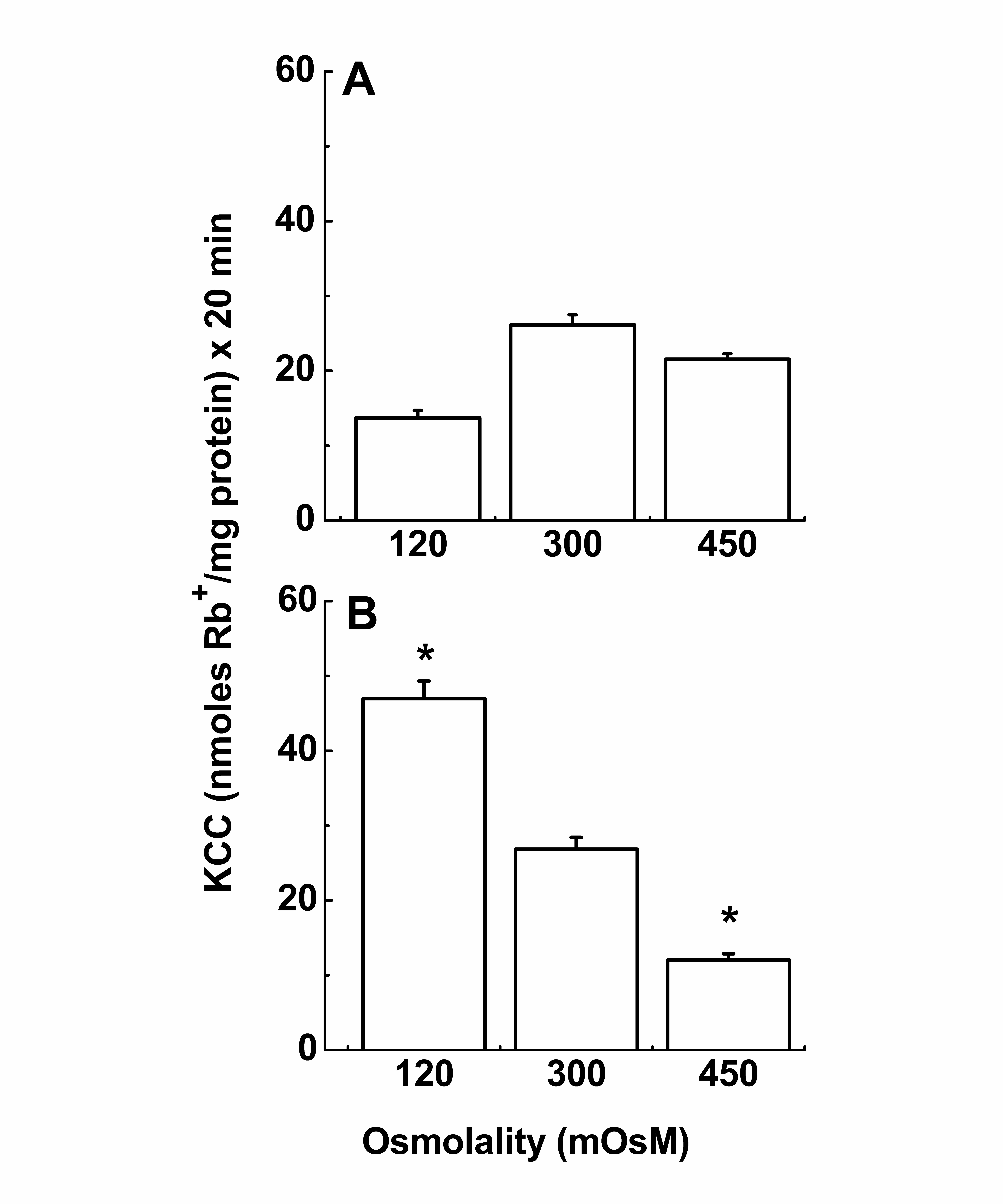
Figure 3. External Na+ influences KCC sensitivity to osmoregulation. VSMCs (passage 15) were cultured as described in Materials and Methods and fluxed in the absence (Panel A) or presence (Panel B) of external Na+ at different osmolalities. Changes in osmolality in solutions containing Na+ were achieved by using NaCl and NaSf, whereas NMDGCl and NMDGSf were used for Na+-free media. The difference between Rb+ influx in NMDGCl and NMDGSf is the Na+-independent KCC activity. Ouabain (2 mM) was used during flux alone while bumetanide (30 µM) was present during preincubation and flux. As shown in panel B, KCC activity is differentially regulated according to changes in osmolality (*p<0.05). In contrast, in Na+-free media (A), KCC activity did not behave as expected with changes in osmolality (see panel B). Data are mean ± standard error from two independent experiments (n = 6 individual determinations per condition).
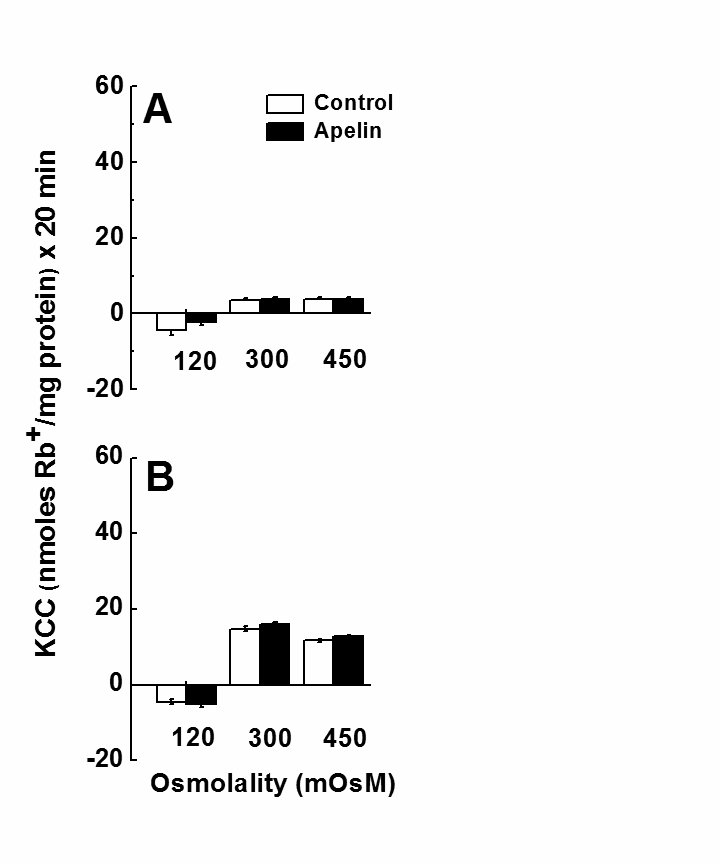
Figure 4. Effect of apelin-13 on KCC activity in primary cultures of rat aortic vascular smooth muscle cells (VSMCs) at various osmolalities in the absence of Na+. The difference between Rb+ influx in NMDGCl and NMDGSf is the Na+-independent KCC activity. (A) Contractile VSMCs (passage 3), and (B) Synthetic VSMCs (passage 39). Rb+ influx was determined as described in Materials and Methods, and at different osmolalities in Na+-free solutions. Osmolality changes were achieved by adding NMDGCl and NMDGSf. Ouabain (2 mM) and bumetanide (2 µM) were present in preincubation and flux solution. Apelin-13 (1 µM) was added during flux. Data are mean ± standard error of two independent experiments with n = 6 independent determinations for each condition.
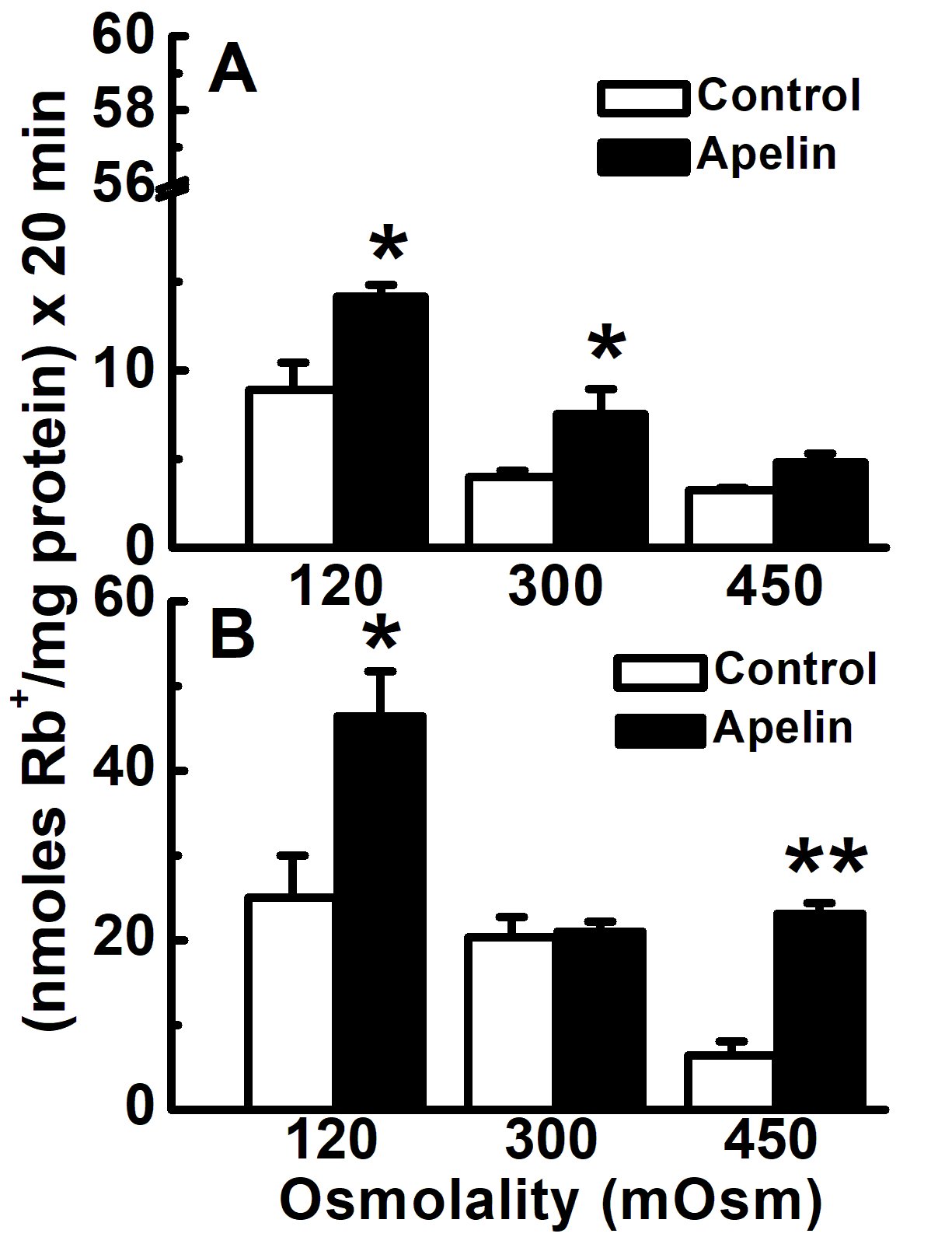
Figure 5. Acute effect of apelin-13 on KCC activity in contractile and synthetic VSMCs as a function of the external osmolality and in the presence of Na. Contractile (passage 3) (Panel A) and synthetic (passage 7) (Panel B) VSMCs, were grown as described in Materials and Methods until subconfluence. Cells were then washed and exposed to different osmolalities (Hypotonic: 120 mOsM, isotonic: 300 mOsM and hypertonic: 450 mOsM) in the preincubation and flux media. Ouabain (2 mM) was present during flux alone while bumetanide (30 µM) was present during preincubation and flux. Apelin-13 (1 µM) was present during flux. NaCl and NaSf salts were used to change the osmolalities of the external media. In panel A, apelin-13 treatment increased KCC in contractile VSMCs at all the osmolalities tested albeit it was statistically significant under the hypotonic and isotonic but not hypertonic conditions. In panel B, apelin-13 treatment in synthetic VSMCs statistically significantly increased KCC activity under hypo and hypertonicity but not under isotonic conditions. Data are mean ± standard error of three independent experiments with n = 3 individual determinations per condition. *p<0.05 and ** p<0.01, apelin vs control group.
Chronic Apelin Effect on KCC in Activity in Synthetic VSMCs under Hypotonic, Isotonic and Hypertonic ConditionsThe lack of apelin effect in synthetic VSMCs under isotonic and hypertonic conditions could also be due to insufficient exposure time. Thus, the apelin effect in hypotonic, isotonic, and hypertonic conditions was studied under chronic exposure to the drug as follows: KCC activity was evaluated in synthetic VSMCs (passage 13) grown until sub-confluency in serum rich media and then apelin-exposed for 0.5, 1, 12, 18 and 24 h before flux in hypotonic, isotonic, and hypertonic media. After the chronic treatment with apelin in isotonic media, cells were then preincubated for 10 min, as described in Materials and Methods with the exception of the media osmolality that varied according to the flux protocol, and then incubated in the corresponding hypotonic, isotonic, or hypertonic media to measure Cl--dependent Rb+ influx for 20 min in the presence of 1 µM apelin. As shown in Fig. 6A, apelin treatment statistically significantly increased KCC in hypotonic media with respect to its control at all incubation times (** p < 0.01 and * p < 0.05). Apelin incubation in isotonic media (Fig. 6B) initially inhibited KCC activity at 0.5 h followed by a gradual increase in response that was statistically significant at 18 h (* p < 0.05) followed by an inhibition that was at the level of the control at 24 h. Similarly, in hypertonic conditions (Fig. 6C), the response was bimodal, an inhibition of KCC at 0.5 h (*p < 0.05) followed by a gradual increase that was significant at 12 h (*p < 0.05), and subsequent reduction to the baseline at 24 h (* p < 0.05). Fig. 7 summarizes this information where the apelin response during the course of 24 h is shown as an average for each tonicity tested, next to the corresponding averaged controls for each tonicity. A polynomial fit shows a decrease in KCC activity both in the absence and presence of apelin as a function of osmolality, in agreement with results in Fig. 2 for KCC activity, and with previous findings on apelin and the effect of osmolality [22].
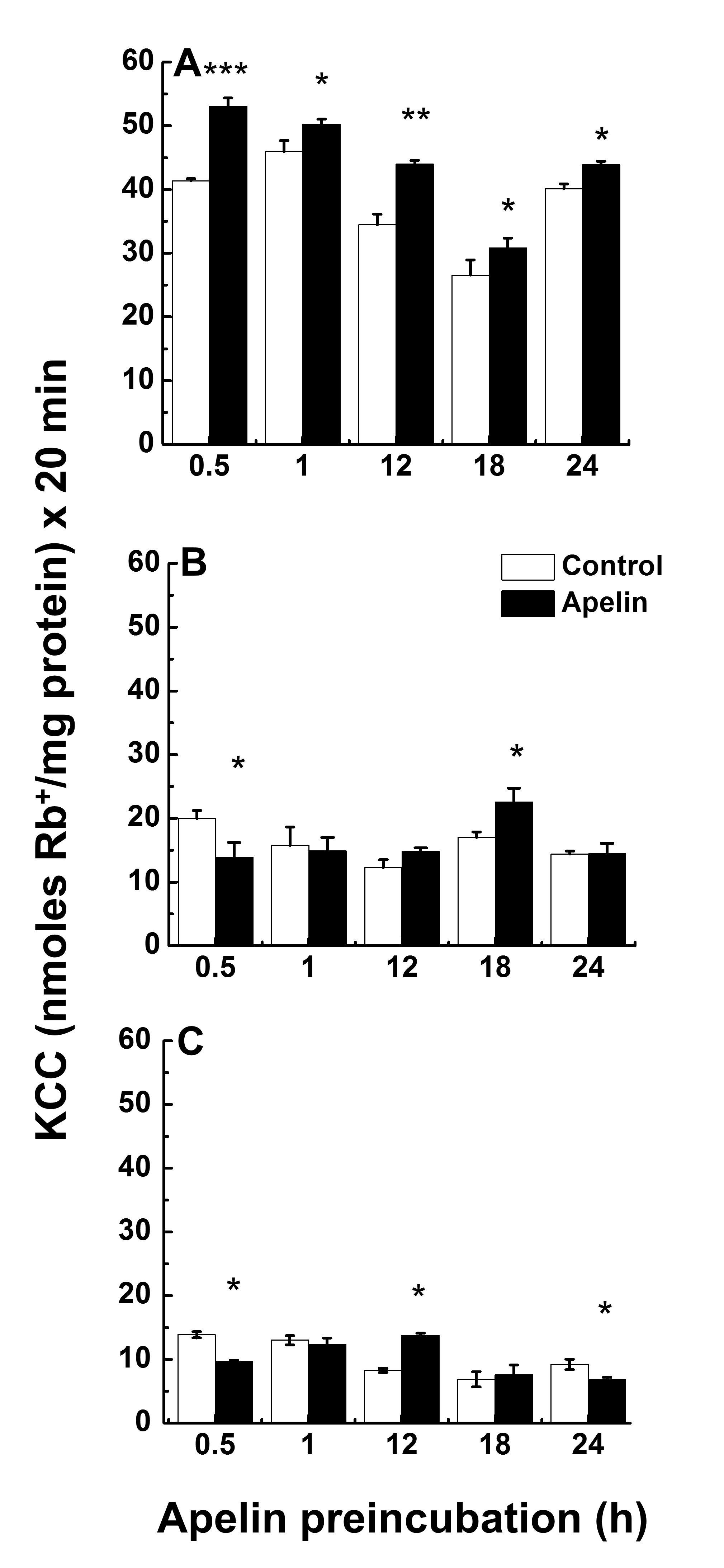
Figure 6. Metabolic pathways potentially impacted by the induced change in chronic hydration based on changes in serum metabolomic profile between Week 1 and Week 6. Pathway impact was analyzed for known compounds that changed by 2-fold or more in serum. Metabolomic pathways identified only in serum when normalized relative to the pooled Week 1 samples are marked with an asterisk (*). Bold font denotes 2-fold or greater change observed in this analysis. Red and blue font represent 2-fold or greater increases and decreases between Week 1 and Week 6, respectively. Metabolic pathways potentially impacted are shown enclosed in a box. Metabolic intermediates that changed are shown without a box. Hypothesized correlated changes, which might be inferred but were not observed in this analysis, are shown without bold font. Example sources of substrate for the TCA cycle are highlighted in yellow. See Table 3 for detail about metabolic pathways potentially impacted.
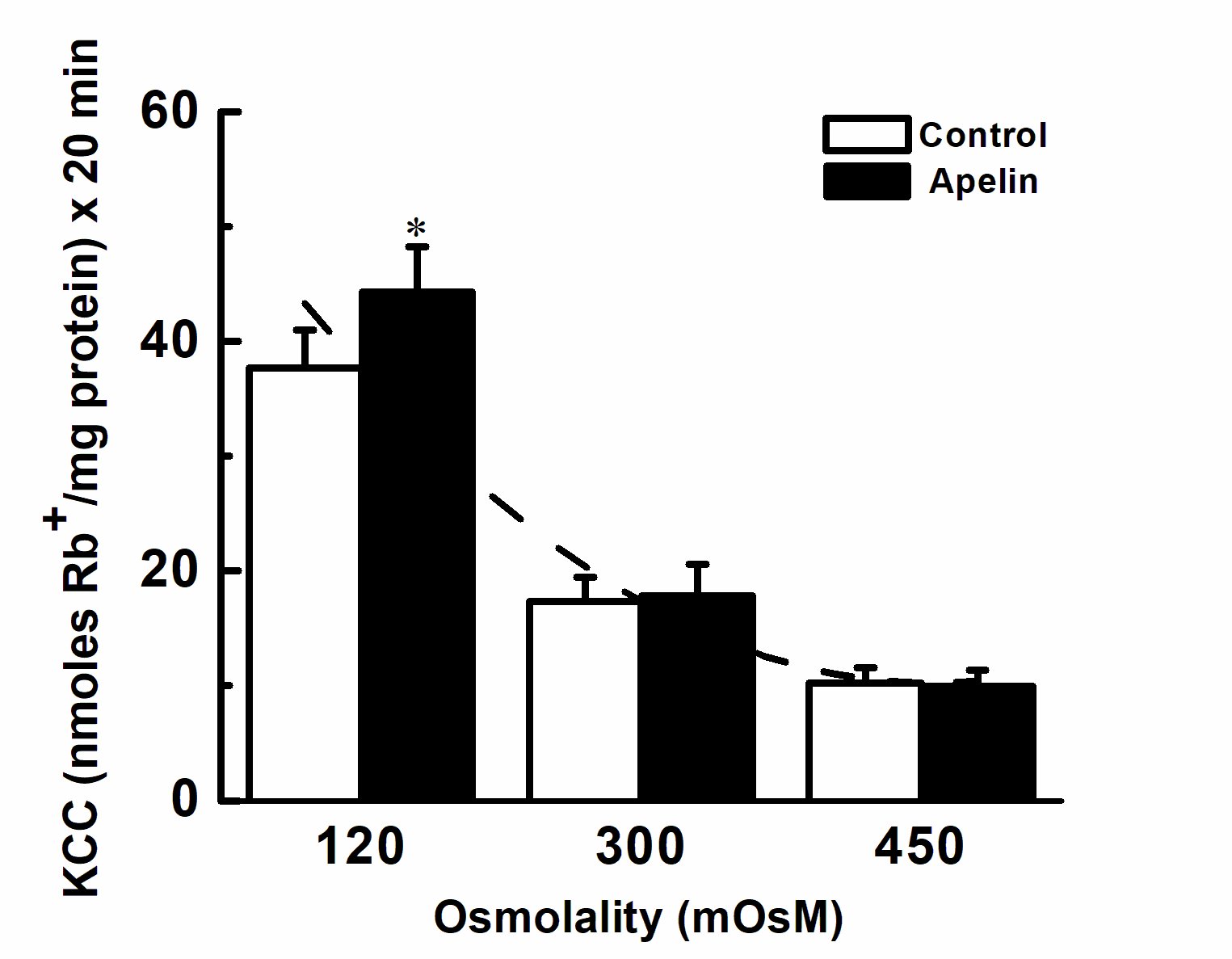
Figure 7. Chronic effect of apelin-13 as a function of osmolality. The average apelin response for each tonicity tested from 0.5 to 24 h is shown with respect to its averaged control (see Fig. 6 for experimental conditions). A polynomial fit shows a reduction in KCC activity both in the presence and absence of apelin as a function of osmolality, whereas apelin statistically significantly stimulated KCC activity in hypotonic but not in iso- or hyper-tonic conditions (*p<0.05, n = 15 individual determinations for both control and apelin for each tonicity).
Osmolality and Apelin Regulation of KCC in Synthetic Phenotypes
Synthetic VSMCs were used to further assess whether the apelin-induced KCC activity was cell volume-dependent. To this end, two observations were important: 1) KCC could still be stimulated by apelin under hypertonic conditions (in which basal KCC activity is normally abrogated) (Fig. 5A and 5B), and 2) The stimulation by chronic apelin treatment in hypo, iso and hypertonic conditions (Fig. 6A-C, respectively). Thus, the effect of cell volume manipulation by shrinking and swelling was tested to explain differences between acute and chronic apelin effects and its response in preswollen and preshrunken cells to assess its potential role in edema and hypovolemic shock, respectively.
With this objective in mind, several conditions were tested in a series of experiments to determine whether changes in osmolality and their sequence, i.e., a) cell swelling (hypotonic at 120 mOsM), b) cell shrinkage (hypertonic at 450 mOsM) followed by swelling (hypotonic at 120 mOsM), c) shrinkage alone (hypertonic at 450 mOsM), and d) swelling (hypotonic at 120 mOsM) followed by shrinkage (hypertonic at 450 mOsM), could alter the effect of chronic apelin treatment on KCC activity in synthetic VSMCs. Cell volume was not measured in this study, but external osmolality was modulated to such an extent that corresponding cell volume changes must have ensued.
Fig. 8 shows the effect of 6 h of chronic apelin treatment on synthetic VSMCs in isotonic medium, as described in Material and Methods, followed by 10 min preincubation of the cells in hypotonic (120 mOsM), or hypertonic (450 mOsM) medium in the absence of apelin, and then 20 min during flux in hypotonic (120 mOsM) medium in the absence (control) or presence of apelin (experimental) (for further details see figure legend). We chose 6 h for these experiments as the shortest incubation time expected to induce activation of KCC by apelin, previously seen for the chronic incubations in Fig. 6 and 7 above. Indeed, Fig. 8 shows that under hypotonic conditions/cell swelling, 6 h of chronic apelin treatment increased KCC activity by 54 % with respect to its control (*p < 0.05), whereas osmolality shifts from hypertonicity to hypotonicity (shrinkage followed by swelling) increased KCC activity by 74 % (**p < 0.01 with respect to its control), a 20 % additional increase as compared to the hypotonic conditions with no osmolality shift.
Inversely, for shrinkage alone (450 mOsM) (condition c, above) and swelling (120 mOsM) followed by shrinkage (450 mOsM) (condition d, above) the following was found: In (d), apelin failed to stimulate KCC activity, whereas in (c, shrinkage alone), apelin stimulated KCC, albeit not statistically significantly (data not shown), as previously seen in Fig. 5B for the acute effect of apelin where the statistically significance was higher (** p < 0.01, apelin vs control group).
The increase in KCC activity elicited by apelin in preshrunken and subsequently swollen VSMCs progressing from early to later synthetic states is shown in Fig. 9. Apelin stimulation of KCC (apelin vs. controls) was stronger in early synthetic VSMCs (passage 7, ap < 0.01) as compared to later stages (passages 13 and 20, bp < 0.05). These data indicate that the apelin-mediated activation of KCC was a function of the phenotypic transition of VSMCs (passage 7-20) (cp < 0.05 between passage 7 and 13, and dp < 0.001 between passage 7 and 20). Conversely, in pre-swollen (120 mOsM) VSMCs subsequently shrunken in 450 mOsM media, apelin failed to stimulate KCC activity, although it did stimulate KCC, albeit not statistically significantly, in shrunken cells (data not shown), see Fig. 5B for the acute effect of apelin. Similarly, when pre-swollen VSMCs at different passages were transferred to hypertonicity, there was a tendency for apelin to decrease KCC at the lowest passage and increase it as the cells progressed into later synthetic states, although results were not statistically significant (data not shown). Hence, VSMCs progression from early to later synthetic states affects the apelin stimulation of KCC activity and is dependent on whether the cells are subject to osmolality changes.
To further understand how apelin regulates KCC, the signaling pathways prominent in each phenotype were studied.
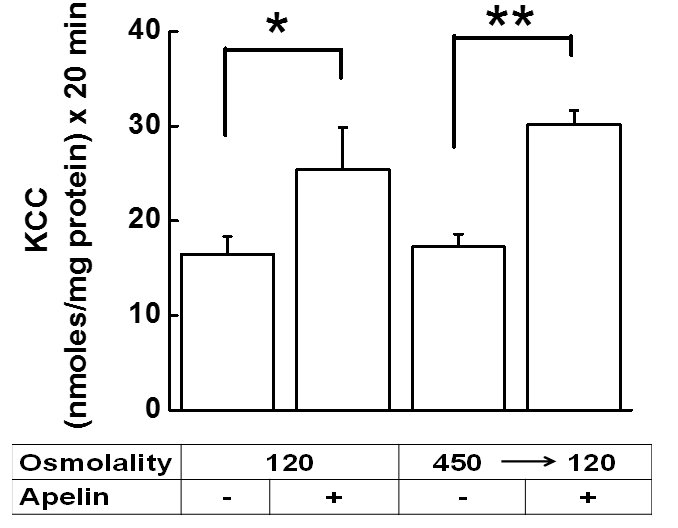
Figure 8. Chronic apelin-13 treatment reveals enhanced KCC sensitivity in synthetic VSMCs through changes in external osmolality. KCC-mediated Rb+ influx was measured in synthetic VSMCs (passage 7). Cells were grown as described in Materials and Methods and after reaching subconfluence, apelin-13 was added for 6 h in the treatment group and was present for an additional 20 min during flux, whereas the control group had no apelin-13. Ouabain (2 mM) was present during flux alone, while bumetanide (30 µM) was present during preincubation and flux. Apelin-13 stimulated KCC activity in hypotonic condition (120 mOsM), and also in preshrunken cells that were first exposed to hypertonic solution (450 mOsM) during preincubation (10 min) and then to hypotonic solution (120 mOsM) during flux (20 min). Paired t-tests were performed between apelin-13 treated and control group for each condition. The apelin-13 mediated response in preshrunken cells was statistically significantly higher (** p<0.01) compared to the swollen cells (*p<0.05). Data are mean ± standard error from 2 independent experiments, each done in triplicate (n = 6 independent determinations per condition).
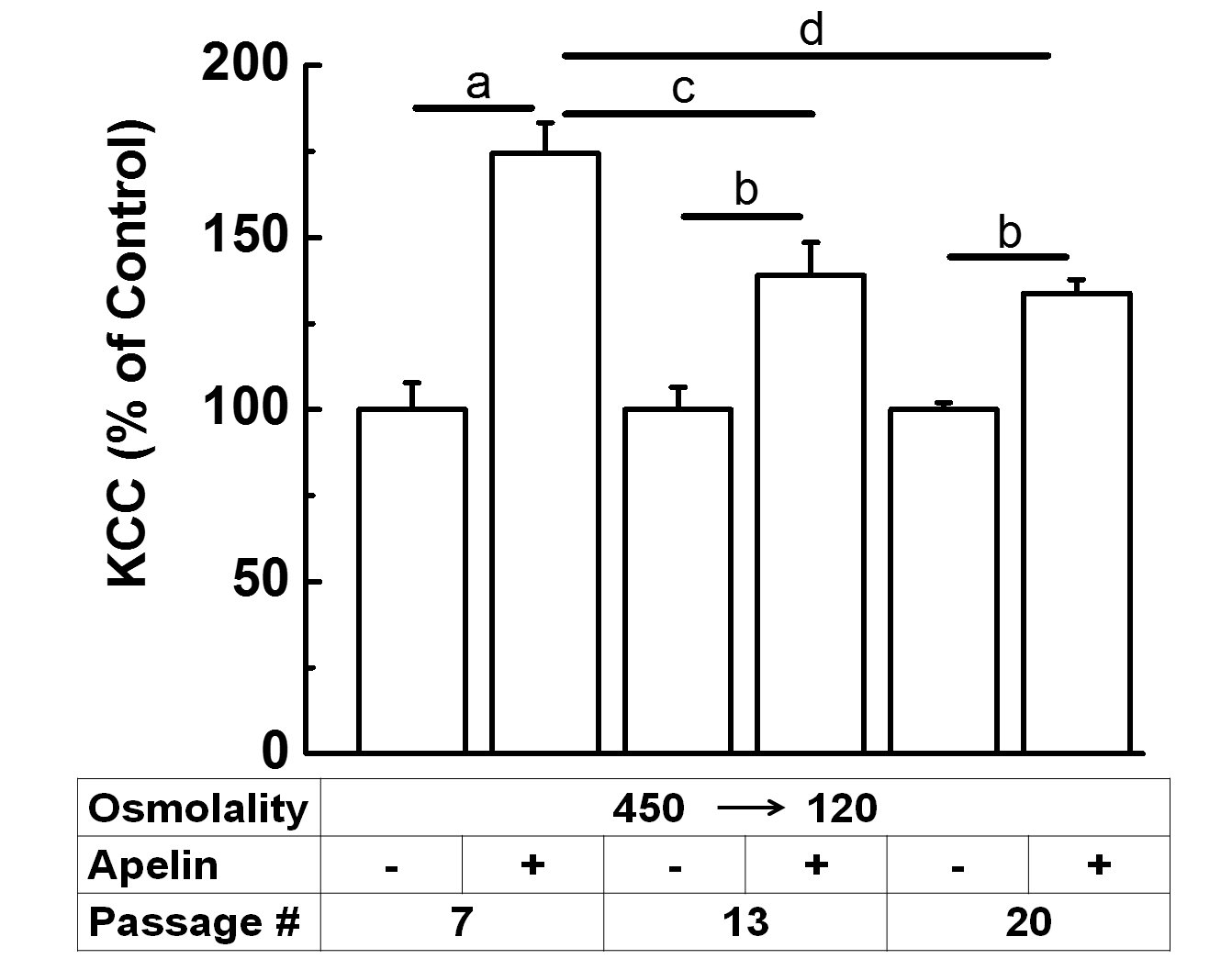
Figure 9. Apelin-13 response in preshrunken synthetic VSMCs is passage-dependent. Data show the apelin-mediated increase in KCC activity (expressed as % of the respective controls) in pre-shrunken cells at passages 7, 13, and 20 that were subsequently swollen (450 120 mOsM). Cells were grown as described in Material and Methods and after reaching subconfluence, apelin-13 was added for 6 h in the treatment group and was present for an additional 20 min during flux, whereas the control group had no apelin-13. Apelin-13 stimulated KCC activity in preshrunken cells first exposed to hypertonic solution (450 mOsM) during preincubation (10 min) and then to hypotonic solution (120 mOsM) during flux (20 min). Paired t-test showed ap<0.01, apelin-13 vs. control group, for passage 7, and bp<0.05, apelin-13 vs. control group, for passages 13 and 20. Data are mean ± standard error from three independent experiments (n = 6 individual determination for each condition). Statistical analysis of apelin-mediated increase with respect to passages done by One-Way ANOVA followed by Tukey’s test showed a statistical significance of cp<0.05 between passage 7 and 13, and dp<0.001 between passage 7 and 20.
Apelin Regulation of KCC by NO-mediated Signaling and Potential Role of oxLDLTo assess whether apelin upregulates KCC through the NO/cGMP/PKG pathway, contractile VSMCs at passage < 4 were used to ensure PKG was present (Fig. 10). VSMCs were cultured until sub confluence and then serum-starved for 24 h, according to the manufacturers recommendations. Prior to flux measurement, cells were then incubated with 2.5 µM KT5823, a potent inhibitor of PKG, for 10 min in preincubation media followed by an additional 30 min during the flux, and in the presence and absence of apelin (1 µM). The Cl--dependent Rb+ influx for each condition was normalized with respect to the control so that analysis of similar experiments and comparison between the different effectors and signaling pathways could be done. As shown in Fig. 10A, the inhibitor alone did not statistically significantly affect the basal KCC activity. Apelin treatment of contractile (PKG-positive) VSMCs statistically significantly increased KCC activity by 87 % compared to the basal control (ap < 0.05). When contractile VSMCs were incubated in the presence of KT5823 (Fig. 10A), the apelin-mediated increase in KCC was prevented suggesting regulation by the NO pathway (bp < 0.001 apelin + KT5823 vs. apelin).
Because oxLDL modulates progression of atherosclerosis by impairing the NO-mediated pathway in contractile VSMCs [36, 37], oxLDL was tested in a preliminary experiment (Fig. 10B) for any effect on KCC activity in the presence and absence of apelin. Rb+ influx was measured in contractile VSMCs (passage 4) treated for 24 h with 8 μg/mL oxLDL and then followed by a 40 min incubation during flux, according to the manufacturer’s recommendations, and in the presence of 1 μM apelin. Apelin treatment increased KCC by 2-fold, while oxLDL inhibited the baseline KCC by over 70 % (cp < 0.01, n = 3). Furthermore, the oxLDL-mediated inhibition of KCC was reversed to the value of the untreated control by apelin treatment (Fig. 10B).
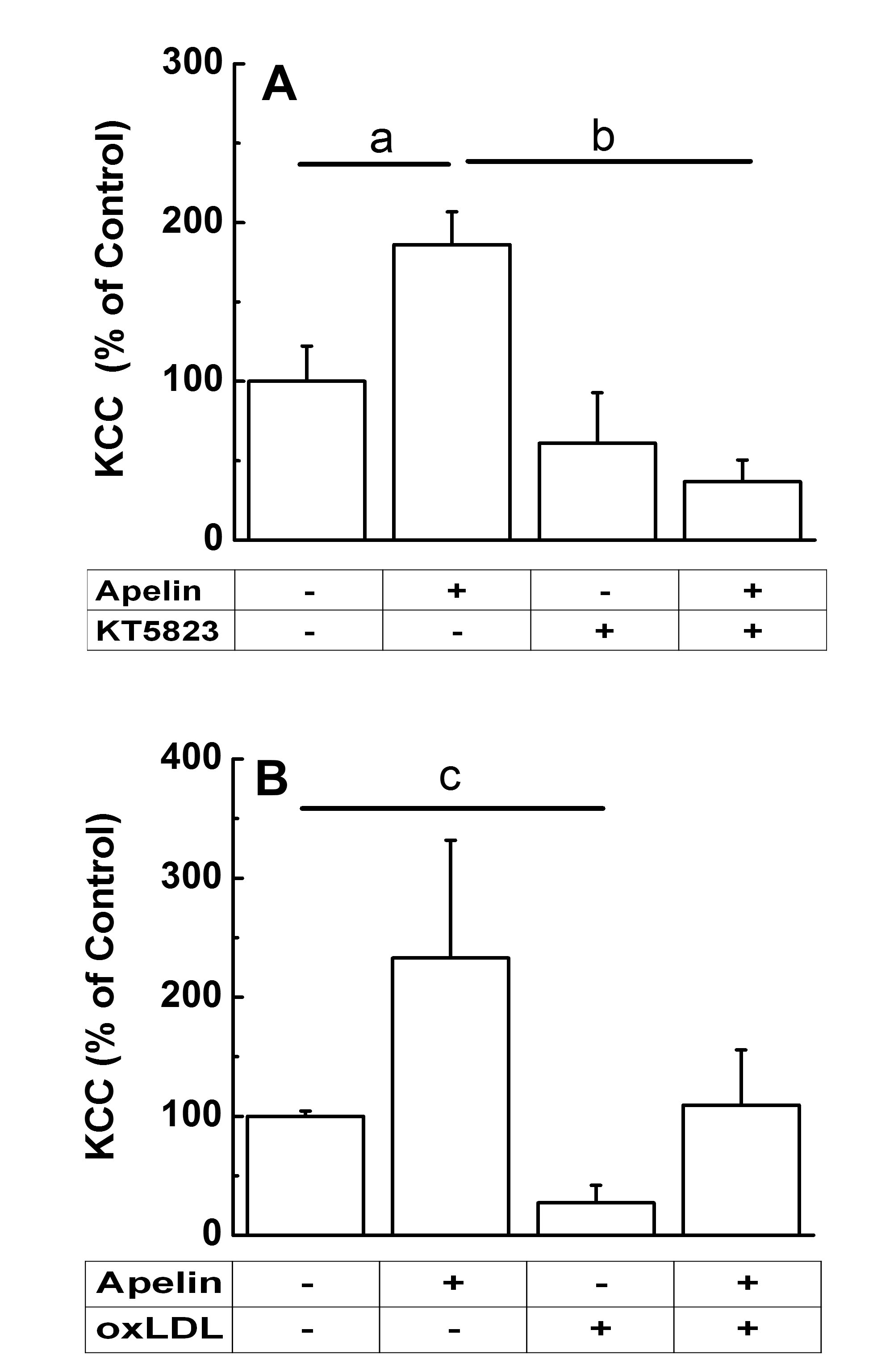
Figure 10. Apelin-13 effect on K-Cl cotransport by the NO-mediated pathway. Contractile VSMCs were cultured as described in Material and Methods and according to the manufacturers’ instructions for the inhibitors tested. (A). KCC-mediated Rb+ influx from 24 h serum-starved contractile VSMCs (passage 1 and 3) was measured in the presence or absence of 1 µM apelin-13 (30 min incubation during flux) and 2.5 µM of KT5823, an inhibitor of PKG (10 min preincubation and 30 min flux). Data were normalized with respect to the control and represent means ± standard errors of two independent experiments each done in triplicates, n = 6 per condition. One-Way ANOVA with post-hoc Tukey test showed ap<0.05 apelin-13 vs. control group, and bp<0.001 apelin-13 + KT5823 vs. apelin-13. (B). Acute treatment with apelin-13 appears to rescue chronic oxLDL-mediated inhibition of KCC activity. KCC-mediated Rb+ influx, from 24h-serum-starved contractile VSMCs (passage 4), was measured in the presence or absence of 4 μM apelin-13 (40 min incubation during flux) and 8 μg/mL oxLDL. Apelin dose-response studies (1-4 μM, n = 8, results not shown), showed no statistically significant difference on KCC activity, consequently the highest concentration was used to test the effect of oxLDL. Sub-confluent cells were incubated with oxLDL in serum-free media for 24 h. Cells were then washed with BSS and the Rb+ influx assay was done as described in Materials and Methods. Apelin-13 was added during flux to determine whether it caused an acute effect on chronically oxLDL-treated cells. Data are mean ± standard deviation of a preliminary experiment, n = 3 individual determinations per conditions. Paired t-test showed cp <0.01 oxLDL vs. control.
Apelin Regulation of KCC by PI3K/Akt and MAPK-mediated Signaling PathwaysThe effect of selective inhibitors of the PI3K/Akt and MAPK/ERK1/2 pathways on KCC activity was assessed in the presence or absence of apelin (Fig. 11). Sub-confluent synthetic VSMCs (passages 6-8) were serum-starved for 24 h prior to flux measurement and incubated for 10 min with 10 µM of either LY294002 (Fig. 11A) or PD98059 (Fig. 11B), selective inhibitors of PI3K/Akt and ERK1/2, respectively, and according to the manufacturers recommendations. Then, cells were further exposed to these inhibitors for 40 min during the flux period, again according to the manufacturers recommendations, in the presence or absence of apelin. Results were normalized with respect to the control. Fig. 11A and B shows that acute apelin treatment activated KCC in synthetic VSMCs by 142 % (ap < 0.01, n =6, apelin vs. control). However, in the presence of inhibitors, VSMCs were no longer responsive to apelin-mediated KCC activation (bp < 0.05, apelin-13 + LY294002 vs. apelin-13, and apelin-13 + PD98059 vs. apelin-13). Note that neither inhibitor affected baseline KCC activity. These results indicate that apelin/APJ regulates KCC via the PI3K/Akt and MAPK/ERK1/2 pathways.
A summary of the signaling pathways, that result in KCC upregulation by apelin as defined by the selective inhibitors used, is shown in Fig. 12. The inhibitors KT5823 (Fig. 10A), PD98059, and LY294002 (Fig. 11A and B, respectively) blocked KCC activation in the presence of apelin, whereas the inhibitory effect of oxLDL on KCC (Fig. 10B) was reversed to baseline levels by apelin.
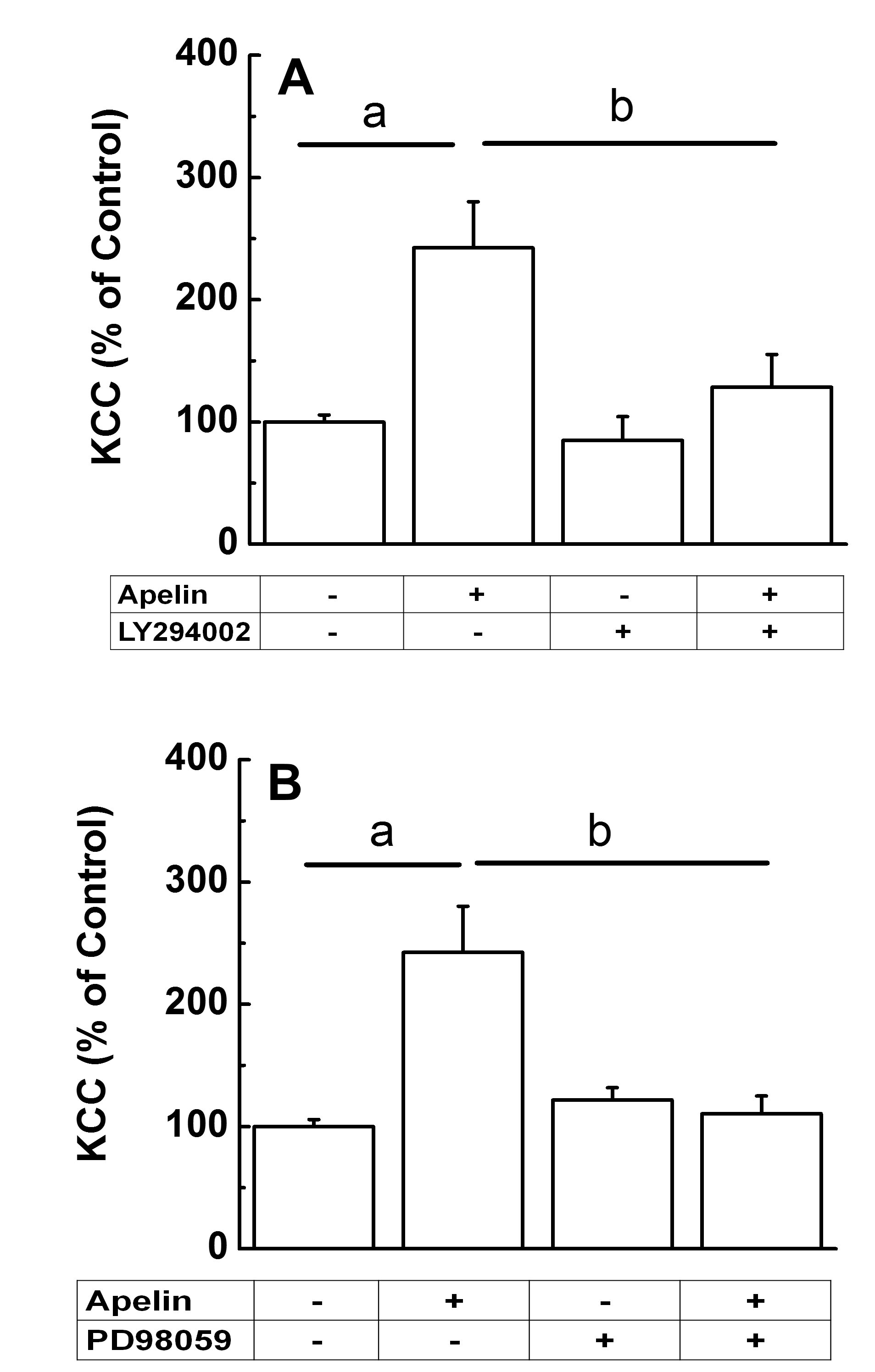
Figure 11. Apelin-13-mediated activation of KCC occurred through a PI3K/Akt and MAPK pathways in synthetic VSMCs. (A) PI3K/Akt pathway. KCC-mediated Rb+ influx by 24 h serum-starved synthetic VSMCs (passages 6-8) was measured, according to Materials and Methods and the inhibitors manufacturers recommendations, in the presence or absence of 1 µM apelin-13 and 10 µM of LY294002, an inhibitor of the PI3K/Akt pathway. LY294002 was present in preincubation and flux media. Apelin-13 was added to the flux solution. One-Way ANOVA with post-hoc Tukey test showed ap<0.01 for apelin-13 vs. control group, and bp<0.05 for apelin-13 + LY294002 vs. apelin-13. (B) MAPK pathway. KCC-mediated Rb+ influx by 24h serum-starved synthetic VSMCs (passage 6-8) was measured, according to Materials and Methods and the inhibitors manufacturers recommendations, in the presence or absence of 1 µM apelin-13 and 10 µM of PD98059, an inhibitor of the MAPK pathway, in preincubation and flux. One-Way ANOVA with post-hoc Tukey test showed a,bp<0.01 for apelin-13 vs. control group, and apelin-13 + PD98059 vs. apelin-13. For both (A) and (B), experimental outcomes were normalized to the baseline control. Data are mean ± standard error of two independent experiments, n = 6 individual determinations per condition.
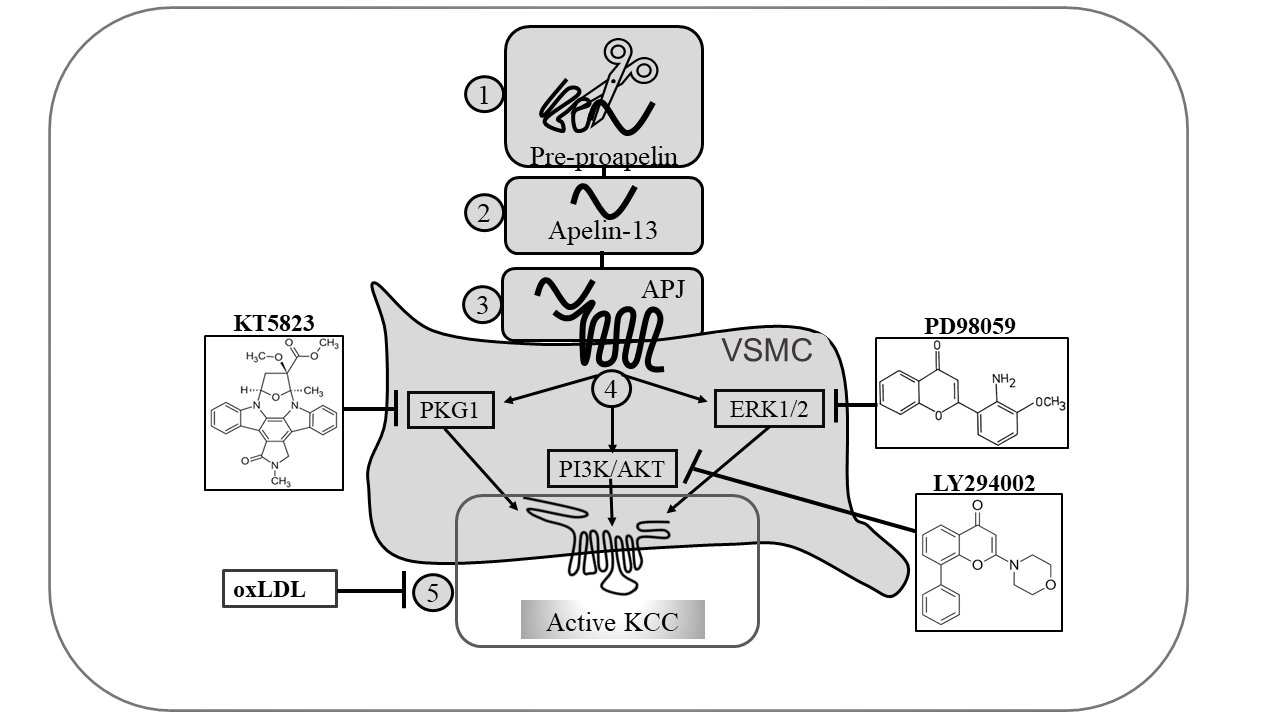
Figure 12. Proposed signal transduction pathway of KCC regulation by apelin-13 in contractile and synthetic VSMCs. Pre-proapelin (1) is converted into apelin-13 (2), binds to its receptor APJ (3), and elicits three signaling pathways (4). In contractile VSMCs, oxLDL inhibits KCC activity (5) whereas apelin-13 sustains this mode of cotransport via the NO/sGC/PKG-mediated pathway. In synthetic VSMCs, apelin-13 activates KCC via the PI3K/Akt and MAPK/ERK1/2-mediated pathways. The drug KT5823 blocks the NO-mediated pathway, whereas LY294002 and PD98059, the PI3K/Akt and MAPK pathways, respectively.
Coordinated Regulation of the Cellular/Body Fluid Osmotic Balance by KCC/NKCC and Apelin/VasopressinFig. 13 is a model representing the activities of KCC, and NKCC, and the relative concentrations of apelin and vasopressin as a function of osmolality. The diagram represents the transporters and hormones interacting with their counterparts according to a Yin-Yang mechanism to preserve cell and body osmotic balance and fluid homeostasis.
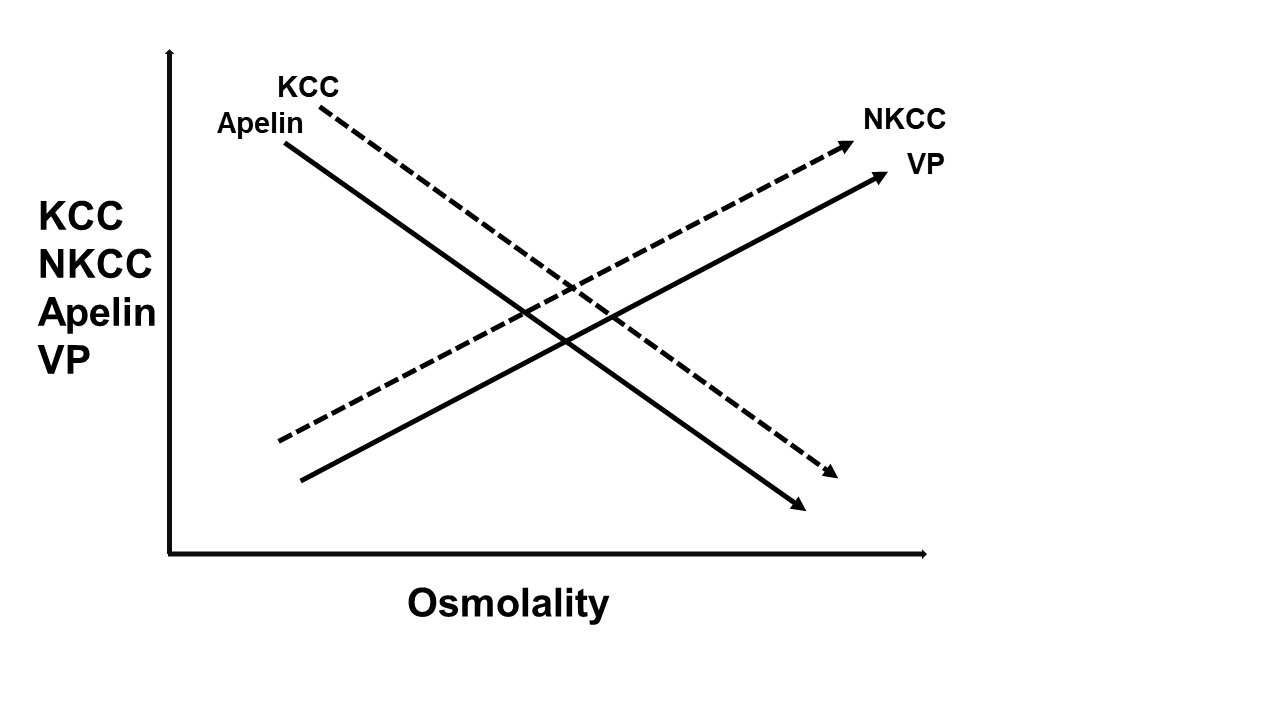
Figure 13. Coordinated Regulation of the Cellular/Body Fluid Osmotic Balance by KCC/NKCC and Apelin/Vasopressin (VP). The model represents the activities of KCC, and NKCC, as defined in this study, and also the relative concentrations of apelin and VP [22] as a function of osmolality. In the model, the ion transporters KCC, and NKCC, function as a Yin-Yang mechanism of cellular ion and volume homeostasis, whereas apelin, a vasoactive peptide, and VP, a neurotransmitter also known as anti-diuretic hormone (ADH), have been described as the Yin-Yang of water balance [11]. Extensive literature supports the present findings indicating that apelin regulates KCC through mechanisms that would result in vasodilation. In contrast, the role of NKCC and VP is to function as vasoconstrictors, and this is also supported by numerous studies. Thus, the role of the two Yin-Yang systems is to work in a coordinate manner to preserve cell volume and body fluid regulation in a tightly controlled manner, and abnormalities in any of their functions can lead to CVD and other pathologies. Such a coordinated system offers numerous variables and, concomitantly, targets for corrective interventions.
Apelin Receptor Expression in VSMCsThe relationships between the APJ receptor which shares identity with the AT1 (angiotensin II (Ang II)) receptor type 1, Ang II, the renin-angiotensin aldosterone system (RAAS), angiotensin converting enzyme 2 (ACE2), the heptapeptide Ang-(1-7), and apelin, a powerful inotropic and cardiovascular protective peptide, as indicated above, have been and are subjects of intensive studies. This is due to their various roles in CVD, such as the progression of hypertension where Ang II induces cardiovascular remodeling [13, 38], the opposing effects of Ang-(1-7) counteracting the effects of RAAS, and apelin promoting vasodilation by the NO-dependent pathway ([16], and references therein; [13, 39, 40]). Furthermore, upon binding to its receptor, APJ, apelin triggers a plethora of signaling cascades (such as the NO/cGMP/PKG, PI3K/Akt and MAPK/ERK 1/2), and a coordinated cardio-protective multicellular response takes place to repair and prevent further cell damage ([22]; [14], and references therein; [15], and references therein) and to induce changes in protein expression that have proven to be beneficial against CVD ([16], and references therein; [13]).
Migration of VSMCs and their phenotypic transition from contractile to synthetic states are initial steps in the repair of the atherosclerotic endothelium. Failure of VSMCs to switch back to contractile states and instead undergo remodeling, adds to dysregulations in cell proliferation and to the development of atherosclerosis ([2]; [3], and references therein; [41, 42]).
Common signaling networks regulate the activity of both KCC and the apelin-mediated cardio-protective effects ([4], and references therein; [5], and references therein; [7], and references therein; [15]). Here, evidence suggests that electroneutral KCC in VSMCs responds to apelin via its membrane receptor APJ. Although differences were found in the APJ subcellular localization between synthetic (homogenously distributed) and contractile VSMCs (perinuclear, localization), in general, the apelin receptor was found abundantly expressed in both cell types (Fig. 1A and C). These findings are supported by studies on the specificity of the primary antibody in different cell types by immunohistochemistry and immunofluorescence [29].
Furthermore, newer evidence reveals localization bias of GPCRs making the signaling and regulation complex to explain, and indicating that not all GPCRs are found in the PM which means that the ligand is not needed for activation and translocation to the PM. Some receptors translocate to the PM upon ligand binding, whereas some are ligand-independent and remain in cytoplasmic or nuclear pools. From our studies, it is likely that there is APJ receptor localization bias in contractile vs. synthetic cells, and during maturity or the aging cycle, when more of the receptors translocate to the PM whether or not the ligand is present [43]. It is also possible that in younger VSMCs the anterograde transport of APJ receptors is slow in the absence of ligand and more signaling is occurring from subcellular organelles showing different levels of response and regulation in signal transduction.
Data corresponding to apelin long-term vs. short-term incubation could explain the differences in Rb+ fluxes; and different osmolalities, also changed the apelin-induced KCC response. For instance: In Fig. 4, the total average of apelin-induced KCC response was higher in synthetic vs. contractile VSMCs, regardless of the osmolality. In Fig. 9, early synthetic VSMCs had higher apelin-induced response than the advanced synthetic cells. In Fig. 10 and 11, the net apelin-induced KCC response was higher in synthetic than in contractile VSMCs. In conclusion, the synthetic phenotype response to apelin was higher than in contractile VSMCs under the different experimental conditions.
In addition, the differential distribution of the APJ receptor between contractile and synthetic VSMCs (Fig. 1A, C) could explain their differences in KCC response to apelin. Furthermore, differences in protein expression levels, potential oligomerization, and subcellular localization of APJ could result in variability of apelin-mediated effects in different VSMCs’ phenotypes. Nonetheless, apelin treatment stimulated KCC, irrespective of the VSMC phenotype. These results suggest a rapid trafficking of APJ to the membrane in order to elicit a comparable response in synthetic and contractile VSMCs. Although further studies will be required to determine whether apelin could change the APJ subcellular localization, it is possible that increased APJ membrane trafficking facilitates apelin signaling in VSMCs ([11], and references therein).
The guanylyl cyclase-dependent PKG is one of the most important regulators of KCC and a determinant of VSMCs contractile phenotype ([4], and references therein; [5], and references therein; [21]; [34]; and references therein). Fig. 1F confirms the presence of PKG in our contractile VSMCs model. In contrast, PKG is absent in synthetic VSMCs ([21]; [4], and references therein; [5], and references therein; [34], and references therein).
Variables Affecting the KCC Response to ApelinAlthough KCC is known to be a Na+-independent transporter in most cells and tissues, including our own studies ([4], and references therein; [5], and references therein; [21]), in the present investigation, KCC activity was measured as the Cl--dependent Rb+ uptake in untreated and 1 µM apelin-treated VSMCs [31]. However, it is possible that under certain conditions, such as hypertonicity in the presence of [Na+]o, a residual presence of NKCC could become a confounding factor in the assessment of KCC activity. Thus, several factors such as [Na+]o, osmolality, length of apelin treatment (acute or chronic), and VSMC phenotype (contractile vs synthetic) were assessed with the purpose to optimize KCC’s determined activity under the various conditions used in this study.
In summary, without [Na+]o, apelin failed to stimulate KCC in both contractile and synthetic VSMCs, as shown in Fig. 4, in addition to a lack of response of KCC to different osmolalities (Fig. 2A, and 4A and B). Furthermore, the negative KCC values in the hypotonic conditions (Fig. 4A and B) have been reported previously by us in VSMCs ([4], and references therein; [5], and references therein), and were associated with various types of K+ channels, some of which not yet identified [44]. Some of these channels are swelling- or mitogen-sensitive and appear to become activated in both Cl- and Cl--free medium becoming confounding factors in the determination of Cl--dependent K+ flux ([4], and references therein; [5], and references therein; [21]; [34], and references therein; [44]). Finally, the higher KCC activity in synthetic but not contractile VSMCs in isotonic and hypertonic conditions (Fig. 4A and B) is commensurate with the coordinate relationship between KCC activity and enhanced synthetic VSMCs migration and proliferation [31].
In the presence of [Na+]o, (Fig. 5-11), contractile VSMCs’ KCC was activated by apelin under all the conditions tested (Fig. 5 and 10), and synthetic VSMCs displayed activation of KCC by acute apelin treatment under hypo- and hypertonic conditions (Fig. 5B), whereas it was activated by chronic apelin treatment at all the osmolalities tested (Fig. 6-9 and 11) but not by acute apelin treatment in isotonic conditions (Fig. 5 vs 6). This lack of activation could suggest that cultured synthetic VSMCs are unresponsive to apelin when the cell integrity is not compromised by changes in osmolality (Fig. 5B). Thus, the [Na+]o dependence of KCC regulation by apelin is striking for KCCs are known to be [Na+]o-independent, and it remains to be determined how [Na+]o modulates the originally described sodium-independent KCC and its response to apelin in synthetic VSMCs.
Apelin Effect on KCC VSMCs at Variable Osmolalities and in Synthetic VSMCs under Isotonic and Hypotonic Conditions
Apelin’s role to modulate KCC activity in response to changes in extracellular osmolality (Fig. 5-9) appears to be dependent on the time of exposure to apelin (Fig. 6). The lack of KCC stimulation at 24 h of apelin incubation could be explained by either proteolytic inactivation of the APJ receptor or because long exposure times may have resulted in its desensitization (internalization). G protein-coupled receptors (GPCRs) like APJ are desensitized following activation by agonists through phosphorylation by members of the GRKs (G-protein coupled receptor kinases) ([2], and references therein), a mode of control of APJ still to be further elucidated.
In both synthetic and contractile VSMCs, apelin treatment resulted in further stimulation under hypotonic/swelling conditions (Fig. 5B and 8-9). Other studies have shown that hypotonic stimuli result in cell swelling and decreased lumen diameters [45]. It is noteworthy that hypotonic conditions also promote KCC activation; thus, all of the above implies that an increase in KCC activity may be responsible for the apelin-mediated cardioprotective effects [46]. This has clinical implications since increased KCC and therefore vasodilation are commonly seen after exposure to hydralazine (HYZ), a vasodilator known to reduce tension of vascular smooth muscle through increase in KCC activity ([5], and references therein). The effect of HYZ can be mirrored by the apelin response under hypotonic stimuli in VSMCs. Altogether our findings highlight increases in KCC activity as important effectors in sustaining normal vascular tone.
Under hypertonic/shrinking conditions, apelin treatment significantly increased KCC activity and bypassed the osmolality-mediated inhibition of KCC in synthetic VSMCs (Fig. 5). To our knowledge, this is the first report of an extracellular stimulus bypassing the osmolality-mediated regulation of KCC activity. Other studies have shown that cell shrinkage results in increased lumen diameters and thus, stimulation of the apelin response in hypertonic conditions (hypovolemic shock) might be beneficial in the maintenance of vessel relaxation in vivo [47].
Consistently, apelin failed to stimulate pre-swollen VSMCs exposed to hypertonic media (shifted from 120 to 450 mOsM) (data not shown). In contrast, when pre-shrunken VSMCs were exposed to hypotonic stimuli (shifted from 450 to 120 mOsM), apelin treatment led to a statistically significant increase of KCC (Fig. 8-9).
Furthermore, because KCC has been shown to play an important role in vasodilation ([4], and references therein; [5], and references therein; [20], and references therein; [21]), these data suggest that treatment with apelin could become an important tool to enhance vasodilation, critical for ameliorating CVD, by its action on KCC function.
Osmolality and Apelin Regulation of KCC in Synthetic Phenotypes
Synthetic VSMCs are implicated in atherosclerosis because of their different properties and regulating factors [2, 48]. Furthermore, mutual regulations between cell volume changes, cell migration, and proliferation have been reported in several cells and tissues ([49]; [50], and references therein; [51], and references therein). However, the mechanism by which the apelin-mediated KCC regulation might or not participate in blood vessel repair or plaque formation and progression has not been investigated in synthetic VSMC. The results in synthetic VSMCs and their dependence on cell volume changes (Fig. 5A and 5B, and 6-7) obtained in this study, could offer some clues about this process.
Interestingly, the extent of activation by acute apelin treatment in hypotonic and hypertonic conditions was considerably less in contractile VSMCs (Fig. 5A) when compared to the synthetic phenotype (Fig. 5B). In contrast, the lack of apelin activation in synthetic VSMCs under isotonic conditions (Fig. 5B) suggest that longer exposure times are needed for apelin to produce its effect (Fig. 6-7). In Fig. 6, the variability in the apelin-dependent-KCC activity as a function of tonicity and incubation time suggests an adaptive modulatory effect of the hormone under such conditions and is commensurate with other reports ([11], and references therein; [14], and references therein; [16], and references therein). In addition, synthetic VSMCs might be unresponsive to apelin when the cell integrity is not compromised by changes in osmolality. In support to our findings, increase in apelin response to hypotonicity correlates with decrease in plasma osmolality and reduction of vasopressin [13, 22]. These studies suggest that apelin secretion regulates body fluid homeostasis maintenance and vascular homeostasis by upregulating KCC activity as an important and unsuspected mechanism thus far. Furthermore, KCC could be considered as an additional factor/target among the latest emerging from the intensive research uncovering the extent and importance of the apelinergic system [13].
The apelin-stimulatory effect on KCC by hypotonicity, either with or without osmolality shift (Fig. 8), strongly suggest that apelin might potentiate regulatory volume decrease (RVD) mechanisms. The fact that VSMCs progression from early to later synthetic states modulated the apelin stimulation of KCC activity, and its dependence on the osmolality (Fig. 9) again supports a relationship between apelin and the regulation of KCC at the molecular level. Based on published reports on the effect of apelin on aquaporin 2 (AQP2) [52], serine and threonine residues within the KCCs carboxyl-terminus may constitute the final targets in the apelin stimulatory effect on KCCs .
Apelin Regulation of KCC by the NO-mediated Signaling and Role of oxLDL, and by the PI3K/Akt and MAPK/ERK1/2-mediated Signaling PathwaysIn contractile VSMCs, prevalent in healthy blood vessels, the (NO)/sGC/PKG pathway is involved in vasodilation ([32], and references therein). In contrast, the synthetic phenotype, predominantly expressed in diseased blood vessels, is characterized by an impaired NO pathway and by a high turnover of the PI3K)/Akt and of the MAPK/ERK1/2 pathways both involved in cell proliferation and migration, and in vascular remodeling and repair [2, 53, 54]. oxLDL is largely detrimental in atherosclerosis as it induces hypercholesterolemia and pro-inflammatory/pro-atherogenic events. The oxLDL impairment of the NO pathway further transforms contractile into synthetic VSMCs by inducing cell proliferation [36, 37, 55, 56]. Apelin effects on proliferation and motility also involve stimulation of the aforementioned signaling cascades ([57]; [14], and references therein).
In this study, apelin activation of KCC in VSMCs was noticeable in early synthetic cells and decreased as cells progressed to higher passages, perhaps due to several factors: 1) Presence of atherosclerotic plaque in vivo under physiological isotonic conditions like in Fig. 5B, 2) Longer apelin incubation time required to see a significant effect (Fig. 6B), and 3) Increased response by switching from hypertonic to hypotonic conditions (Fig. 8 and 9). Furthermore, under isotonic conditions (Fig. 6B), apelin may require longer incubation times when compared to hypotonic (swollen) and hypertonic (shrunken) synthetic VSMCs (Fig. 6A and 6C, respectively).
Our results indicate an intricate overlap among several signaling cascades (NO/cGMP/PKG, PI3K/Akt and MAPK/ERK1/2) to coordinate the apelin-mediated effects on KCC activity. Although selective inhibition of PKG, Akt and ERK1/2 completely prevented the apelin-induced KCC stimulation in VSMCs (Fig. 10-11), further examination will be needed to determine the exact degree of contribution of each signaling cascade and their relevance to sustain vascular tone and endothelial function in vivo. Based on previous studies by us and others, in which PKG expression has proven to be absent in synthetic VSMCs ([4], and references therein; [5], and references therein; [21]; [34], and references therein), it is possible that differential regulation of KCC activity might occur within VSMCs phenotypes, or that different KCC isoforms are at play. Contractile VSMCs could be more dependent on the NO/cGMP/PKG pathway to respond to apelin, whereas in synthetic VSMCs, apelin could elicit its effects mostly through the PI3K/Akt and MAPK/ERK1/2 signaling cascades (Fig. 12). Future studies need to address whether apelin treatment leads to increase of isoform-specific KCC expression/turnover, more efficiently trafficking to the plasma membrane, or posttranslational modifications that promote an increase in KCC activity, such as phosphorylation/dephosphorylation events that greatly determine the activity of NKCCs and KCCs.
Consistent with NKCC and KCC coordinated role in modulating intracellular Cl- concentration, reciprocal modes of activation govern their activity. Increased activity of serine threonine kinases such as members of the WNK family (WNK 1-4) and Ste20 (SPAK and OSR1) enhance NKCC activity and decrease KCC function [58]. Similarly, up regulation of protein phosphatases (PP1 and PP2B) inhibit NKCC and activate KCC [59]. Therefore, it is likely that dephosphorylation of serine and threonine residues within the KCC carboxyl terminus constitute the final events in the apelin stimulatory effect on KCC ([59], and references therein).
Previous studies have shown that apelin and oxLDL have opposite effects on cardiovascular function. In CVD, low levels of circulating apelin have been observed ([14], and references therein). Similarly, it has been shown that low plasma apelin concentrations constitute a risk to develop atrial fibrillation ([15], and references therein), and more recently it has been shown to prevent atrial fibrillation and to exert other beneficial effects in the heart ([60] and references therein). Even though CVD and atherosclerosis are caused and sustained by a plethora of factors, the increasing evidence pointing to apelin administration as a remarkable protective therapy against CVD and atherosclerotic lesions ([60] and references therein) is fully supported by our observations. Apelin treatment could restore the oxLDL-inhibitory effect on KCC activity in contractile VSMCs (Fig. 10B), thus suggesting that oxLDL and apelin counteract each other in the regulation of KCC activity in VSMCs. Further studies in this direction are needed to validate the strength of this enticing preliminary data.
High levels of oxLDL have been shown to increase KCC1 mRNA expression [19], whereas oxLDL inhibited KCC activity in our studies (Fig. 10B). The former could be part of a compensatory mechanism to restore VSMCs’ migration and normal cell volume.
Concerning the mechanism at the molecular level, these results could also suggest that apelin/APJ may regulate KCC via the NO/cGMP/PKG or PI3K/Akt and MAPK/ERK1/2 pathways not only by modifying its activity via phosphorylation/dephosphorylation events, but perhaps through interaction of the receptor via dimerization ([14]; and references therein), and thus through potential allosteric effects, which may affect the KCC conformation itself. This could also explain the antagonistic effect of oxLDL and apelin.
This study has focused on the potential effectors responsible for the maintenance of cellular/organismal homeostasis via the coordinated regulation of ion transporters (NKCC and KCC) by neuro-vasoactive peptides, such as apelin and vasopressin [11, 22], and specifically, on the mechanism of action of apelin on KCC regulation given the overlap with apelin’s signaling pathways.
The model proposed in Fig. 13 is supported by the findings shown in Fig. 2, and 5-11 of the present study, and summarized in the figure. The model implies that KCC regulation by apelin is important for volume regulation in a tightly controlled manner for vascular homeostasis that is essential to avoid vascular remodeling ([11], and references therein; [23]), and for the maintenance of cardiovascular function through vasodilation and consequently, amelioration of CVD.
This work also sheds new insights into the regulatory mechanism of KCC and apelin in COVID-19 patients as mentioned in the introduction section. Apelin-stimulated KCC activity could confer benefit during early stages of COVID by preventing fluid accumulation in patients’ lungs through cellular and organ osmoregulation, and thus further lung injury or cardiovascular complications. Therefore, targeting KCC can be a part of therapeutic regimens to stop disease progression and reduce the pandemic death toll.
KCC is involved in ionic balance and cellular homeostasis. Apelin and its receptor are also implicated in fluid homeostasis ([22]; [11], and references therein; [16], and references therein; [13]). However, the mechanisms by which apelin promotes fluid and cellular homeostasis through regulation of ion transporters are less understood. This study tested the hypothesis that apelin promotes cellular homeostasis through its action on KCC and by modulating its transport activity. Indeed, this homeostatic mechanism is important for overall vascular function and fluid regulation in COVID-19 patients. Finally, the close association between cell proliferation and volume regulation suggests that KCC activity and its regulation by apelin, are both important to confer protection against atherosclerosis, but also protection against COVID-19-associated mortality. A better understanding of apelin effects on KCC will help design a more powerful therapeutic approach to treat atherosclerosis-linked cardiovascular diseases. Furthermore, these studies suggest that apelin secretion regulates body fluid homeostasis maintenance and vascular homeostasis [22] by upregulating KCC activity as an important and thus far unsuspected mechanism, and that KCC could be considered as an additional factor/target among the newest ones emerging from the intensive research areas uncovering the extent and importance of the apelinergic system [13].
The technical assistance of Kathleen Leonard in the initial phase of this project is gratefully appreciated.
Author contributions
N.C.A. conception and design of research; N.S. performed the experiments with initial help from N.C.A. and P.K.L.; N.S., N.C.A., and P.K.L., analyzed data; N.C.A. and N.S. drafted the manuscript; N.C.A., P.K.L., and N.S. edited and revised the manuscript; N.C.A. and P.K.L. approved final version of the manuscript.
Part of this work has been previously presented at the Experimental Biology Annual Meeting and published as abstracts in scientific meetings and as part of N.S.’s dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, 2014.
Funding SourcesFunding support was from the Biomedical Sciences PhD program (N. Sharma), the Pharmacology & Toxicology Department, and Wright State University Foundation for the Cell Biophysics Laboratory, Wright State University (Dayton, OH).
Statement of Ethics
The authors have no ethical conflicts to disclose.