

Review - DOI:10.33594/000000635
Accepted 27 April, 2023 - Published online : 26 May, 2023
1Institute of Cytology, Russian Academy of Sciences, Saint-Petersburg, Russia, †Alexey Vereninov passed away in July 2022.
Many data show that K+ ions are essential for cell proliferation. In this brief review, we summarize our own studies and literature data that characterize the relationship between modulations of intracellular K+ content and the intensity of cell proliferation. Using flame emission assay we compared the transport of monovalent cations in transformed cells and in human mesenchymal stem cells in growing cultures, as well as during stress-induced cell cycle arrest and transition from monolayer (2D) to three-dimensional (3D) spheroids. A decrease in the intracellular content of K+ (evaluated as the ratio of the content of K+ in cells to the mass of cellular protein) associated with the accumulation of G1 cells in the population and accompanied by a stop in proliferation was revealed. The relationship between intracellular K+ and initiation of cell proliferation was further analyzed in human blood lymphocytes (HBL) as a model for the transition of cells from quiescence to proliferation. In HBL stimulated to proliferate, the content of K+ in cells increases during the transition G0/G1/S, accompanying the growth of small lymphocytes into blasts. Cellular water content, calculated from buoyant cell density, is higher in proliferating HBLs and in Jurkat leukemic T cells than in resting HBL. Available data suggest that intracellular K+ is important for successful cell proliferation as the main intracellular ion involved in the regulation of cell volume during the transition from quiescence to proliferation, and high K+ content and associated high water content in the cell are a characteristic feature of cell proliferation and transformation.
This article is in memory Alexey A. Vereninov (1932-2022). He conducted his research in the field of cellular physiology at the Institute of Cytology RAS in Saint-Petersburg. A.Vereninov was always greatly interested in the issues of monovalent ion transport and, in particular, the role of K+ in cell viability. The most significant result of his work over the past decade was the computer program he developed for calculating the balance of the transmembrane ion fluxes in animal cell both in the steady state and in transient processes. Until the last days of his life, A. Vereninov remained devoted to science and did not stop thinking about plans for future study. This brief review of studies aimed at elucidating the mechanism by which K+ control the cell proliferative activity is one of the latest, which was inspired and conceived by A. Vereninov.
Ion transporters and channels that control cellular concentrations of monovalent ions are important for cell growth and proliferation. Early studies linking transmembrane ion fluxes to cell proliferation were performed on T-lymphocytes and fibroblasts in culture [1, 2]. Since then, monovalent ions, in particular K+, as the dominant intracellular ion and a major contributor to the resting membrane potential, have been identified as an important regulator of cell proliferation in both normal and cancer cells.
First, attention was paid to the rapid ion changes in response to growth factors. It has been established that ion movements associated with the activity of ion channels, cotransporters, and pumps are necessary events in a rapid receptor-mediated cellular response to a growth-promoting signal [3-5]. In addition, monovalent ions have been found to be involved in the long-term cellular response that accompanies the transition of cells from a resting state to continuous proliferation and transformation [6-10]. A decrease in the intracellular concentration of K+ due to blocking the Na,K-ATPase pump slows down cell proliferation, stopping cells in the G1 phase of the cell cycle [6, 11]. At present, it is generally recognized that Na+ and Cl– affect intracellular pH, membrane potential, and the concentration of calcium ions in the cytoplasm and are involved in a network of signaling events that control the activity of regulatory proteins of the cell cycle [12, 13]. Cellular Cl- is involved in the hyperpolarization of the cell membrane during the G1/S transition [14]. It has been shown that K+ channels contribute to the regulation of the cell cycle in different cells [15-17]. It has recently been suggested that K+ may be involved in the "pluripotency signaling network" in human pluripotent stem cells [18].
This brief review article, summarizing our own research, will focus on the mechanisms by which, under physiological conditions, intracellular K+ may be involved in maintaining cell proliferation. Our concept of K+ involvement in the regulation of cell proliferation is based on studies of K+ transport (including K+ content in cells and K+ transmembrane influx) and cell proliferation status in cell culture. In particular, in the cultures of transformed cells, a peculiar decrease in the K+ content, associated with a slowdown in proliferation, was revealed. This finding was further analyzed in human endometrial mesenchymal stem/stromal cells during stress-induced senescence and in normal human lymphocytes stimulated to transition from a resting state to the cell cycle and DNA synthesis.
Our earlier studies on the relationship between ion homeostasis and cell proliferation were concerned with the ion changes during the maintainance of growing cell cultures. The K+ and Na+ contents and inward ion fluxes were measured by the flame emission technique [19]. In our studies, for the quantitative determination of ions in a cell, we normalized the analytically measured content of ions in a cell to the content of cellular protein in the same culture. In cell biology, such an assessment of the content of ions or other components in a cell is widely used. Indeed, there are considerable difficulties in estimating intracellular ion concentrations (i.e. cell ion content per cell water content) in monolayer cultures due to problems with experimental evaluation of cell water in attached and asynchronously growing cells. First, in proliferating cell cultures, a decline in the intracellular K+ content evaluated as the ratio of the K+ content to the cell protein mass (mmol K+ per 1 g of protein) was revealed: the growth of the cell culture and the transition from a sparse to confluent monolayer were accompanied by a decrease in the K+ content from 0.9-1.0 mmol/g to 0.6-0.68 mmol/g [4, 20]. The data were obtained on transformed cells of different origin with a high proliferative index (Table 1). The decrease in the K+ content in the cells was parallel to the increase in the monolayer density and occurred under optimal cultivation conditions: for experiments, the cells were sparsely seeded into dishes and every two days the culture medium was replaced with fresh DMEM medium containing 10 % calf serum. The density-dependent decrease in K+content in proliferating cultures was associated with the accumulation of G1 cells in the population and slowing down proliferation and indicated a relationship between intracellular K+ and cell proliferation rate [4]. This is also evidenced by the data obtained on human endometrial mesenchymal stem cells [23] as well as on cells in suspension culture, such as human blood lymphocytes (HBL) and T leukemia cell Jurkat [24] (Table 1).
Shigella are Gram-negative, non-motile, facultative anaerobic bacteria that infect the epithelium lining the terminal ileum, colon, and rectum in the gastrointestinal tract. Shigella is transmitted through feco-oral contamination from human-human transmission. These enteropathogenic bacteria are classified into four species (Shigella dysenteriae, Shigella flexneri, Shigella boydii, and Shigella sonnei) with multiple serotypes each [35].
The decrease in intracellular K+ content (evaluated as the ratio of the cell K+ content to the cell protein mass) in proliferating eMSCs was not caused by long-term cultivation: in sparse and confluent culture in which the cells were seeded on the same day, but with different densities, and the intracellular ion contents were assayed on the 3rd day after seeding, the cell K+ content was lower in a culture with high density than in sparse culture (Table 1, Fig. 1С). The principle conclusion is that when eMSCs are maintained in culture under optimal conditions, changes in the cellular K+ content are not due to the cultivation time, and the lower K+ content in long-term cultures is associated with the a higher cell density in the monolayer.
The content of K+ in cells depends on the cell age: in early-passage (“young”) eMSCs it is higher than in late-passage (“old”) eMSCs (Fig. 1D). Unlike K+, we did not find significant differences in Na+ content in cells of different passages (Fig. 1D). On the whole, these data indicate that during the maintenance of eMSCs in a proliferating culture, the K+ content in cells depends both on the density of cells in the monolayer and on the age of the cell culture.
With the growth of eMSCs cultures, a decrease in the K+ content correlates with the accumulation of cells in the G1 phase of the cell cycle both in the confluent monolayer (Fig 1E) and in late-passage, “old” cultures (Fig. 1F). These data make obvious the link between the cell K+ content and the rate of stem cell proliferation. It is interesting that in growing eMSCs cultures, K+ content modulations occur in the range from 1000 to 700-600 µmol/g, which is higher than the physiological level of cell survival (500-600 µmol/g) accepted by researchers [6, 7,25]. Altogether, these studies show that a higher intracellular K+ content accompanies successful eMSCs proliferation, and the ratio of the cell K+ content to the protein content in the cell can be an informative test for evaluating the functional status of stem cells in vitro.
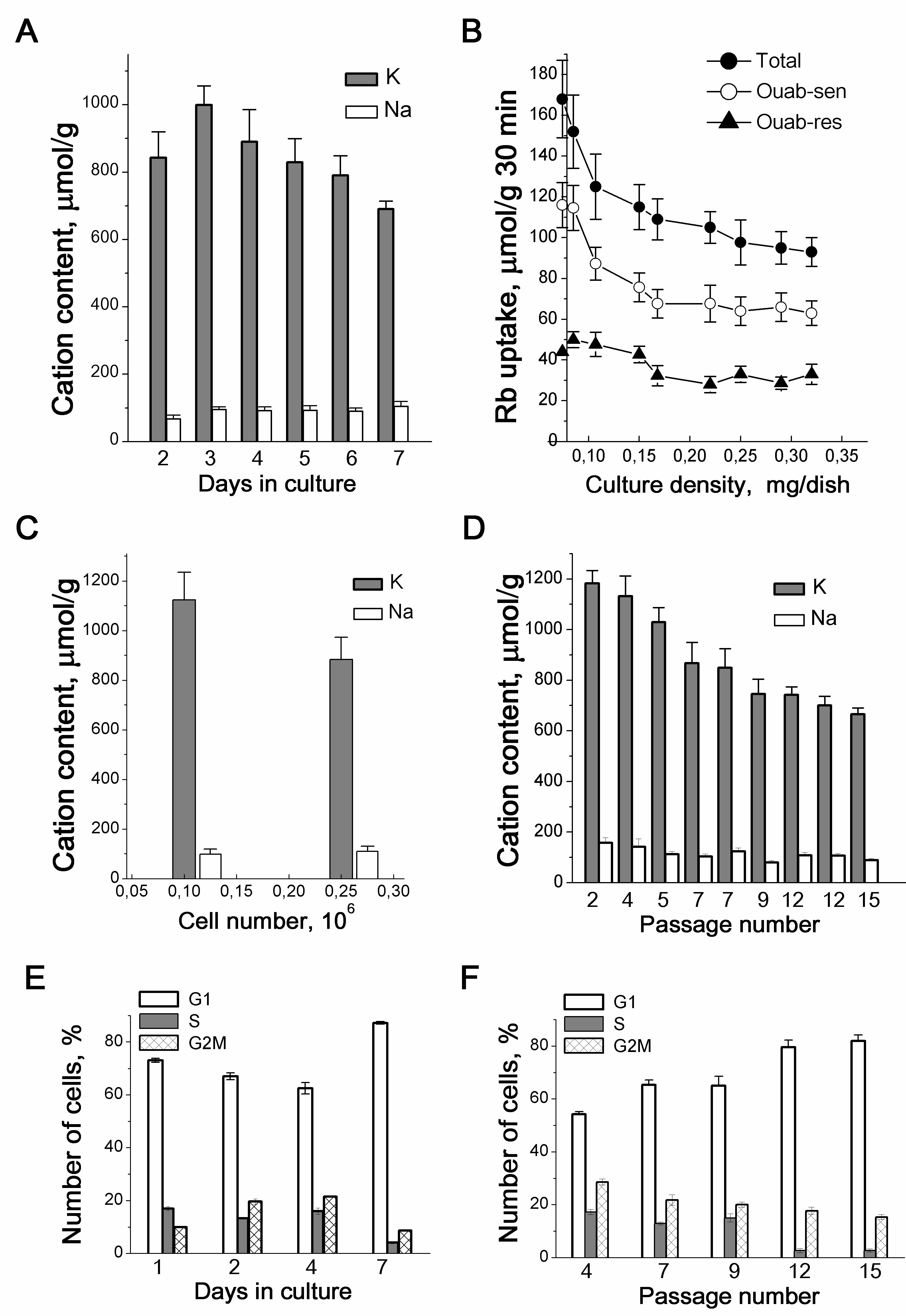
Figure 1. Proliferation-related changes in K+ and Na+ content and Rb+ influxes in growing culture of eMSCs. Cellular K+ and Na+ content (A) and Rb+ influxes (B) depend on the density of eMSCs culture. Total - total Rb+ uptake, ouab-sen - ouabain-sensitive and ouab-res. - ouabain-resistant influxes. C: Increased culture density impacts on the intracellular cation content. Cells were seeded simultaneously at two densities (5x104 and 15 x104 cells per 35 mm dish), and the intracellular cations were estimated on the 3rd day. D: The cell K+ content decreases in eMSCs at late-passages. E, F: The proliferation rate decreases in eMSCs cultures of high density (E) and in cultures with late passage (F). FACS analysis was performed with eMSCs growing within the 7th days in culture (E) or with eMSCs of different passages at the 3d day after plating (F). Measurements of ions were performed by emission flame photometry as described in [23]. Data from 3-5 independent experiments are presented as means+SD for 2-3 cell cultures on the day of experiment. According to [23].
Cellular senescence is defined as an irreversible cell cycle arrest which can be triggered in cells in response to various intrinsic and extrinsic stimuli, as well as developmental signals [26-29]. Despite profound metabolic changes, senescent cells remain metabolically active for a long time. It has been shown that long-term cultivation of human eMSCs is accompanied by the development of premature senescence characterized by inhibition of cell replication [30]. The phenomenon of cellular aging and the senescence development should be kept in mind when the accumulation of a significant cell mass is required for the use of stem cells in clinical practice. In this respect, the parameters of ionic homeostasis of cells in culture are informative for assessing the functional status of stem cells.
Stress-induced eMSCs were used to study ion homeostasis during the development of senescence. Short treatment with 200 μM H2O2of subconfluent eMSCs cultures has been shown to induce senescence [31]. eMSCs subjected to sublethal oxidative stress enter into irreversible cell cycle arrest and exhibit a senescent phenotype including p53/p21/Rb mediated cell cycle arrest, cell hypertrophy, enhanced β-galactosidase staining [31, 32].
After oxidative stress, in arrested eMSCs, K+ influx inhibited by ouabain permanently declines, which indicates a lower activity of the Na/K pump during “early” stress-induced senescence (Fig. 2B). As senescence progresses, cellular seeding content increases and pump-mediated K+ transport is enhances (Fig. 2B and 2C). According to evaluation of the rate coefficient of the Na, K-ATPase pump-mediated transport [33, 34], the increase in ouabain-inhibitabale fluxes in “late” senescent MSCs is not associated with modulation of the propertiesof the ion pump and is due to an increase in the content of Na+ in cells during senescence progression. Stress-induced cell cycle arrest does not affect ouabain-resistant K+ influxes, indicating that senescence is not associated with significant changes in channel-mediated K+ movement across the cell membrane (Fig. 2D).
The most significant observation concerns the K+ content in stress-induced eMSCs: the arrest of proliferation and the progression of senescence are accompanied by a gradual decrease in the cell K+ content from 800-900 µmol/g (cycling and “early” senescent eMSCs) to 500-600 µmol/g (arrested, “late” senescent eMSCs) (Fig. 2A). Premature senescence caused by various stresses is associated with the cessation of cell proliferation. Senescent eMSCs are non-proliferating cells, and the lower K+/protein ratio in such cells is in good agreement with our previous finding: a decrease in this ratio accompanies a density-dependent decrease in the cell proliferation rate [4, 23]. Thus, the ratio of the K+ content to the protein mass in the cell can be used as an informative test of the functional state of stem cells: a high cell K+/protein ratio accompanies successful proliferation of eMSCs, whereas a decrease in this ratio is associated with the development of cell aging.
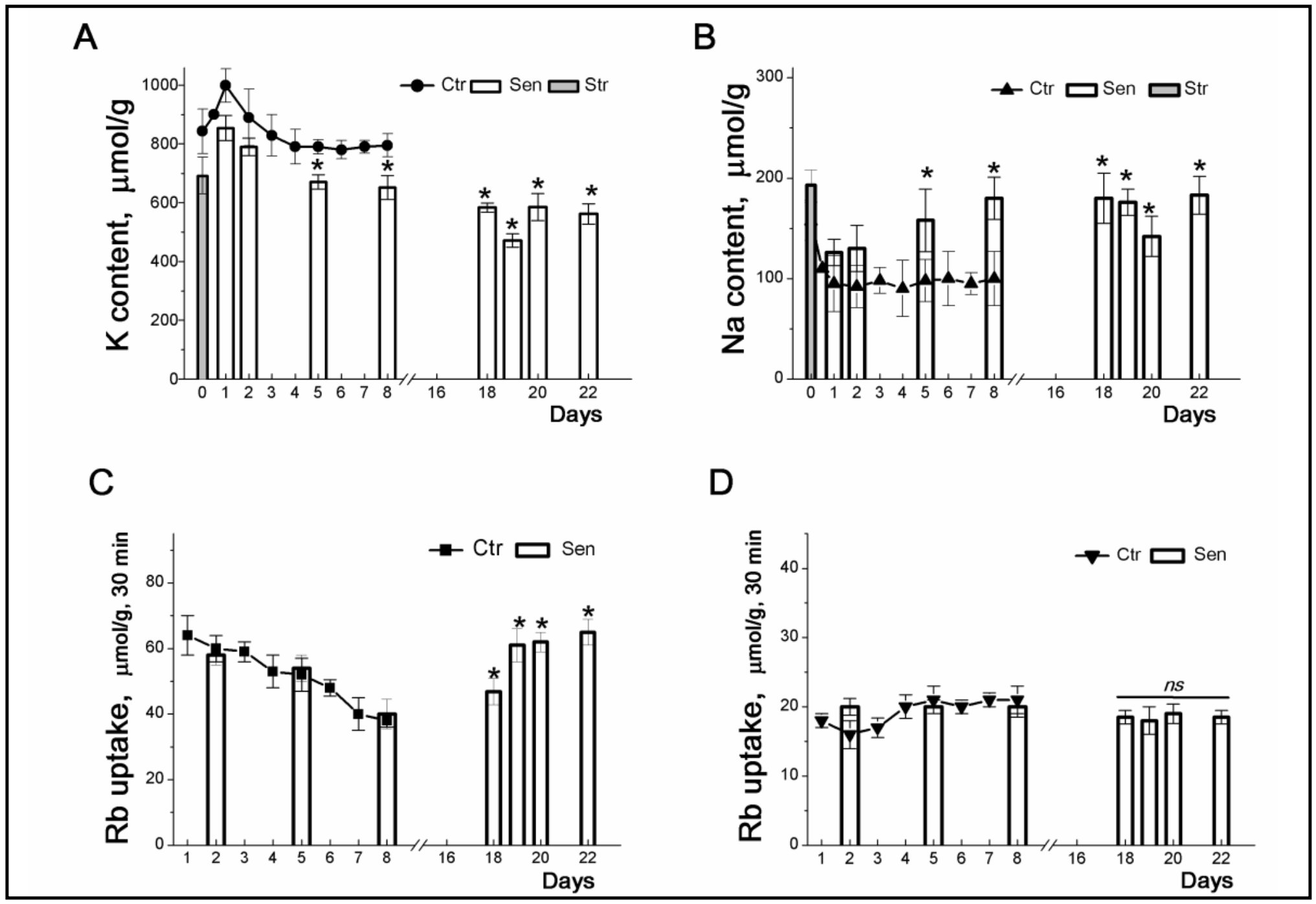
Figure 2. Changes in K+ and Na+ contents and Rb+ influxes during stress-induced senescence in human eMSCs. Cells were seeded in 3-cm dishes (10x104 cells per dish) and on the second day after seeding, some cultures were subjected to 200 μM H2O2 for 1 h followed by H2O2 replacement and cell cultivation under normal conditions. The content of cellular K+ (A, circles) and Na+ (B, triangles up) in cells during culture growth. The content of K+ (A, columns) and Na+ (B, columns) during senescence progression. Grey columns represent cellular content of K+ (A) and Na+ (B) after treatment of cells with 200 μM H2O2. C: Ouabain-sensitive (C, squares) and ouabain-resistant (D, triangles down) Rb+ influxes in cycling MSCs.Ouabain-sensitive (C, columns) and ouabin-resistant (D, columns) Rb+ influxes during senescence progression. For the first 8 day, data are shown as means+SD for six independent experiments performed in triplicate; significant difference between stress-induced and control proliferating cells (Ctr) was calculated using one-way ANOVA with Tukey’s post hoc tests, *p<0.05. For late senescent cells (18-22 days) data are shown as means+SD (n=3). According to [32].
Human eMSCs incorporated into various three-dimensional (3D) structures are more appropriate for clinical trials, however, the cellular phenotype and cell behavior change as the culture environment changes from 2D to 3D configuration [35, 36]. During the transition from 2D to 3D cell culture we observed serious changes in ion content of eMSCs.
Suspended eMSCs detached from the monolayer, accumulate Na+ and have low transmembrane ion gradients: the loss of cell-cell and cell-extracellular matrix cоntacts leads to dissipation of ion gradients between the cell and the extracellular medium (Fig. 3A). When suspended eMSCs are placed in hanging drop conditions and organized into compact 3D spheroids, they restore low Na+ content and have higher pump-mediated Rb+ (K+) influxes as well as the high K/Na ratio (Fig. 3A and 3C). 3D eMSCs are cells arrested in the division cycle and also characterized by a lower K+ content accounting 500-600 μmol/g (Fig. 3A and 3B). Under hanging drop conditions, 3D eMSCs are viable cells: they start the cycle when seeded in a monolayer 2D culture. It is noteworthy that cell adhesion in 2D culture and the transition to proliferation are associated with an increase in the cell K+ content to 700-900 µmol/g (Fig. 3A). Thus, human eMSCs in compact multicellular spheroids are non-proliferating cells, and a lower cell K+/protein ratio is consistent with our assumption that a decrease in this index indicates a decrease in the rate of cell proliferation.
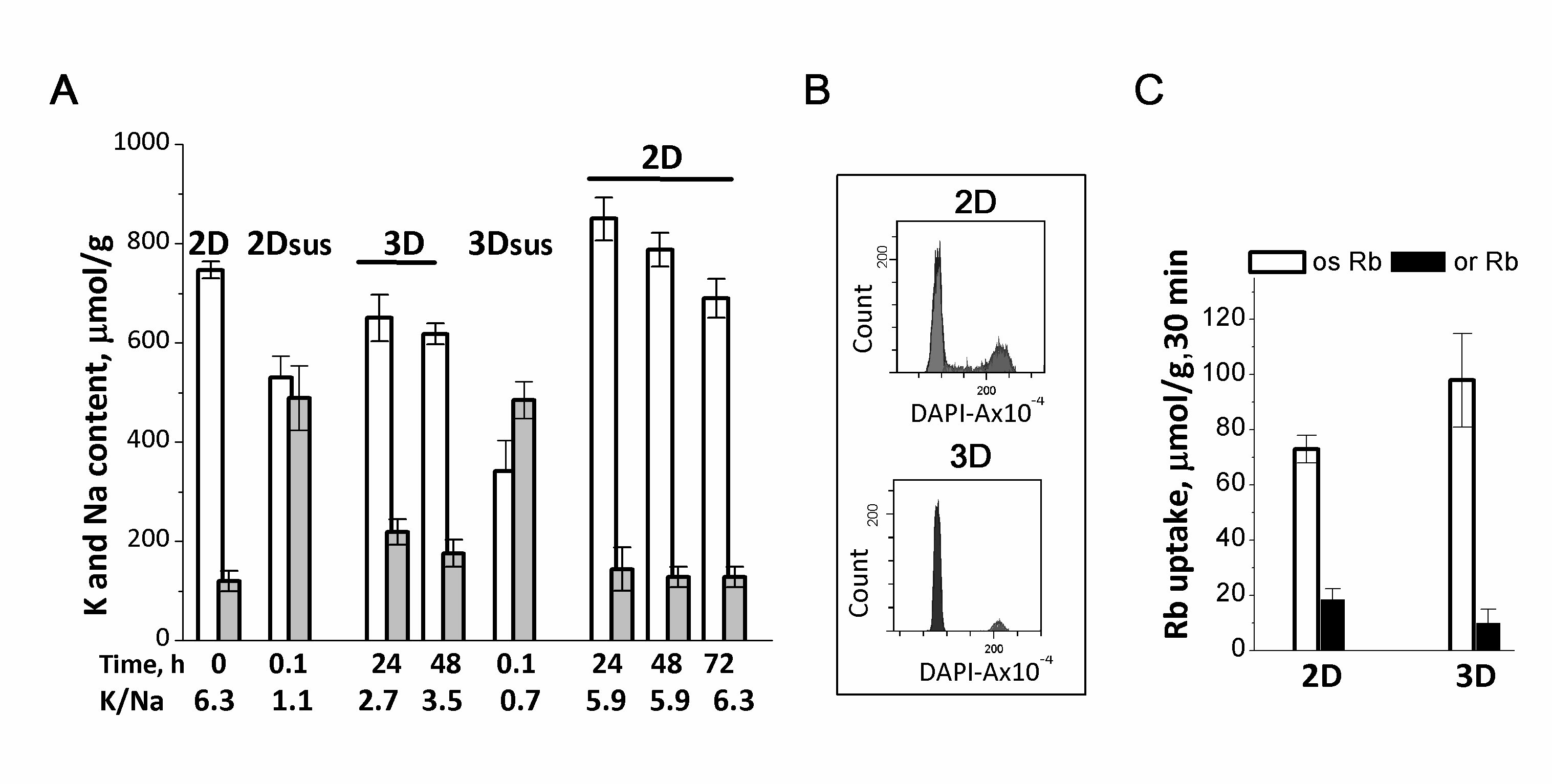
Figure 3. Ion homeostasis of human eMSCs during the transition from 2D to 3D culture. A: 2D eMSCs (passage 11) were taken for experiments as a pre-confluent monolayer, harvested by trypsinization, and maintained in suspension (2Dsus) in DMEM/F12 medium supplemented with 10% fetal calf serum for 0.1-0.5 h. In spheroids (3D), eMSCs have a low Na+ content as compared to cells in suspension (2Dsus). 3D eMSCs were grown in hanging drops at a cell density of 15x103 cells/30 µL of growth culture medium for 48 h, then seeded as a monolayer (2D) and cultivated for a further 72 h. B: Flow cytometry analysis of the distribution of cell cycle phases in 2D and 3D eMSCs. C: Rb+ influxes, ouabain-sensitive and ouaban-resistant, in 2D and 3D eMSCs. Rb+ uptake is presented as µmol/g, 30 min. Data are shown as means + SD, n=3-5 for one experiment out of three conducted using the same protocol. According to [37].
The relationship between cellular K+ content and proliferation was further examined in human blood lymphocytes which are an adequate model for investigating the events underlying the transit of resting cell to proliferation. Human peripheral blood lymphocytes (HBL) are quiescent (G0) cells. Cellular quiescence is actively maintained: certain transcription factors acts as regulators of gene expression patterns that enforce the quiescent phenotype [38, 39]. In quiescent HBL, antigen recognition with appropriate co-stimulation triggers exit from G0 state and further transit through cell cycle (G0→G1/S/G2/M).
In HBL stimulated by PHA, or phorbol ester with ionomycin (PDBu+I), or anti-CD3 with interleukin-2 (IL-2), the initial decrease in K+ content in the first hours is followed by a long-term increase in the intracellular content of K+ [19, 24]. Compared to resting cells, cellular K+ was higher in activated HBL: 500+24 mmol/g after 5 h and 820+56 mmol/g after 48 h (Fig. 4A). The initial decrease in the K+content takes place simultaneously with an increase in the cell Na+ content. At later stages, the content of K+ in the cells increases, while the content of Na+ does not change or slightly increases by the end of the second day, when the cells pass into the G2/M phases.
In resting HBL, IL-2 does not by itself induce the high-affinity receptor for IL-2, cell growth and proliferation, or any changes in cell K+ content. The addition of IL-2 to a culture of competent HBL, pre-treated with non-mitogenic doses of PHA initiated a proliferative response and caused a sustained increase in the cell K+ content over the next 48 h (Fig. 4B). Thus, in activated HBL, a long-term increase in the cell K+ content is associated with IL-2-dependent progression when small T cells are transformed into blasts.
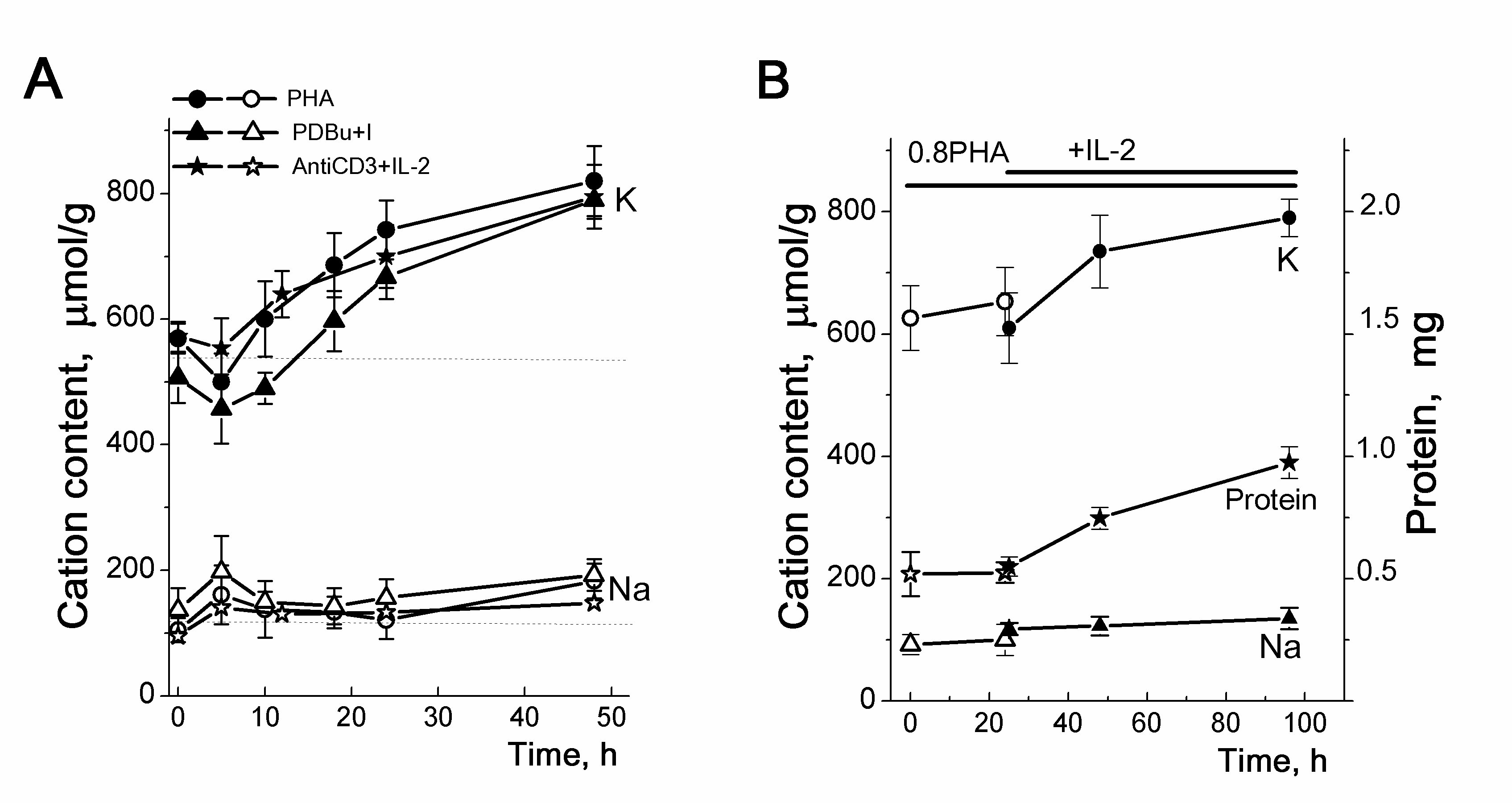
Figure 4. Cellular K+ and Na+ contents in human HBL activated to transition from quiescence to proliferation. A: Changes in the cellular K+ (K) and Na+ (Na) contents in human HBL stimulated by PHA (10 µg/ml), or PDBu (10 nM) with ionomycin (I, 500 nM) or anti-CD3 (3.5µg/mL) with IL-2 (100 U/mL). The contents of cellular K+ (dark symbols) and Na+ (light symbols) were analyzed at definite time points by flame emission photometry. B: IL-2 induces a long-term increase in cellular K+ and cellular protein content in competent HBL. Isolated HBL were incubated with non-mitogenic PHA (0.8 µg/mL) for 20 hours, then IL-2 (100 U/mL) was introduced into cell culture. Data are means D of 5 experiments performed triplicate.]. According to [24].
The relationship between an increase in the intracellular content of K+ and the initiation of cell proliferation is seen in experiments with drugs specific for different steps of the G0/G1/S transit. In mitogen-stimulated HBL, cyclosporine A, which inhibits the production of IL-2 and the initiation of HBL proliferation, prevents both cell growth and an increase in the K+ content of cells (Fig. 5). WHI-P131, which induces apoptosis in some lymphoma cells, targets JAK3 tyrosine kinases and prevents the induction of the IL-2Rα gene in normal T cells [24]. We found that at concentrations that do not affect survival of resting HBL but prevent the initiation of the G0/G1/S transit, WHI-P131 inhibited both growth and increase in intracellular K+ in stimulated T lymphocytes (Fig. 5).
In view of significant changes in the K+ content in HBL during their transition from quiescence to proliferation, it is important to find out whether the intracellular K+ concentration (the K+ content in cell water) changes in this case. Very informative for such a task are studies of buoyant density of cells, which makes it possible to estimate the intracellular water content in cells in suspension cultures [40].Density gradient centrifugation has been used to detect and isolate cells of a particular density [41].
Our experiments show that after centrifugation in a Percoll gradient resting HBL are distributed mainly in the high density region occupying two density intervals with mean values of 1.0645 g/mL and 1.0695 g/mL (Fig. 6A). Stimulation of HBL by PHA or anti-CD3with IL-2 leads to a shift of cell populations towards lower densities: after 48 h two low-density (1.039 and 1.042 g/mL) and two high-density (1.0465 and 1.0545 g/mL) populations are isolated (Fig. 6A and 6B).
Based on these buoyant density estimates, the water content per g of protein for resting and activated HBL can be calculated. In resting HBL, the water content was 5.16+0.19 mL/g in light subpopulation and 4.61+0.07 mL/g in heavy subpopulation (Fig. 6G). The most significant increase in average water content (about 27%) was observed on the first day after activation: after 24 h the water content of the activated cells increased to 6.48+0.56 mL/g (lighter cell population) and to 5.51+0.50 mL/g (heavier cell population) (Fig. 6F). Also, the water content/protein mass ratio was higher in transformed human T cells Jurkat than in quiescent HBL (Fig. 6G). On the whole, these data allow us to conclude that the water content in activated HBL (late G1/S cells) is higher than in resting HBL (G0/early G1 cells).
Simultaneous determination of intracellular K+ content per g cell protein by flame photometry and cell water content per g cell protein by measuring the cell buoyant density as well as cell volume using a Coulter counter in resting and proliferating HBL shows that the change in intracellular K+ content is not followed by a change in the K+ concentration in cell water (Fig. 6C). These data allow us to conclude that there are no significant differences in K+ concentration between quiescent and proliferating HBL. Here the question arises, what is the functional significance of the increase in the intracellular K+ content (estimated as the ratio of the K+ content in the cell to the mass of cellular protein) during the transition from the quiescent state to proliferation and cell transformation.
To elucidate the observed changes in K+ content in cells we asked whether intracellular K+ might be essential for initiation of cell proliferation in the context of cell volume regulation. Cell transition from quiescence to cell cycle and continuous proliferation is accompanied by an increase in cell size: before division, the activated cells are growing and cell volume is significantly increased. The role of volume change in regulation of cell cycle is poorly studied. Meanwhile, in experiments with modulations of osmotic cell balance it was found that the cell cycle progression was delayed in osmotically shrunken cells [42]. From the pump-leak theory of monovalent ion distribution between animal cells and the medium follows that the amount of K+ in cell essentially depends on the amount of so called “impermeant (through cell membrane) anions” sequestered in cell. It is the amount of these anions in combination with Na/K pump that determines the water balance of the cell and the accumulation of K+ in the cells [43-48]. We can suggest that in resting cells (like HBL), which are stimulated to begin multiplication, an increase in protein mass and in volume during G0/G1/S progression is accompanied by an increase in the amount of impermeant anions, which inevitably leads to an increase in the influx of water to restore the osmotic balance of the cell with the environment. Here, K+ as the major cellular osmolyte enters the cell so that an increase in the cell K+ content/protein mass ratio correlates always with an increase in the cell water content/protein ratio. Therefore, in animal cells, the increased K+/protein ratio indicates increased hydration. This means, that despite the lack of significant concentration differences, K+ may be important for initiating cell proliferation as the intracellular ion that participates in regulation of volume and water content in cell. Indeed, many evidence indicate that various K+-transporting systems including the Na/K pump, cation–chloride cotransporters and ion channels are involved in regulation of cell proliferation: their expression and function can be regulated by the cell cycle, and inhibition of K+-transporting pathways in the cell membrane can lead to proliferation arrest [7, 11, 15-17, 49-51].
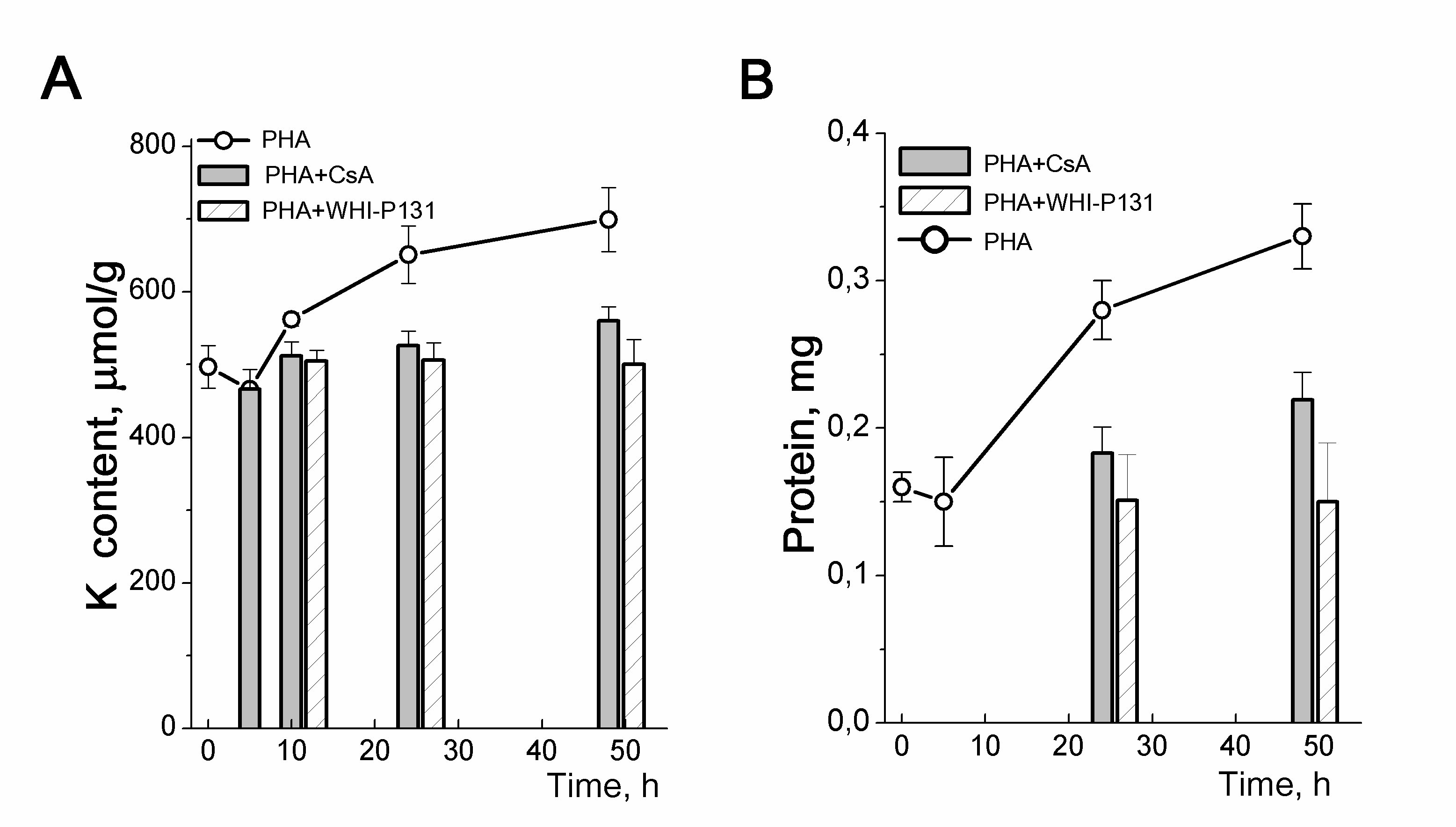
Figure 5. Cellular K+ content and inhibition of proliferation in PHA-stimulated human blood lymphocytes. A: Time-course of K content changes in HBL stimulated with PHA alone or in the presence of drugs specific for the initial (CsA) or late (WHI-P131) stages of HBL activation. B: Anti-proliferative doses of CsA and WHI-P131 inhibit the growth of PHA-stimulated HBL. Cells were cultivated with 10 µg/mL PHA without or with 80 µM WHI-P131 or 1.0 µg/mL CsA for 48 h and were analyzed at definite time of points for K by flame emission photometry and for protein content by Lowry procedure. Data are means D of one representative experiment performed triplicate. According to [24].
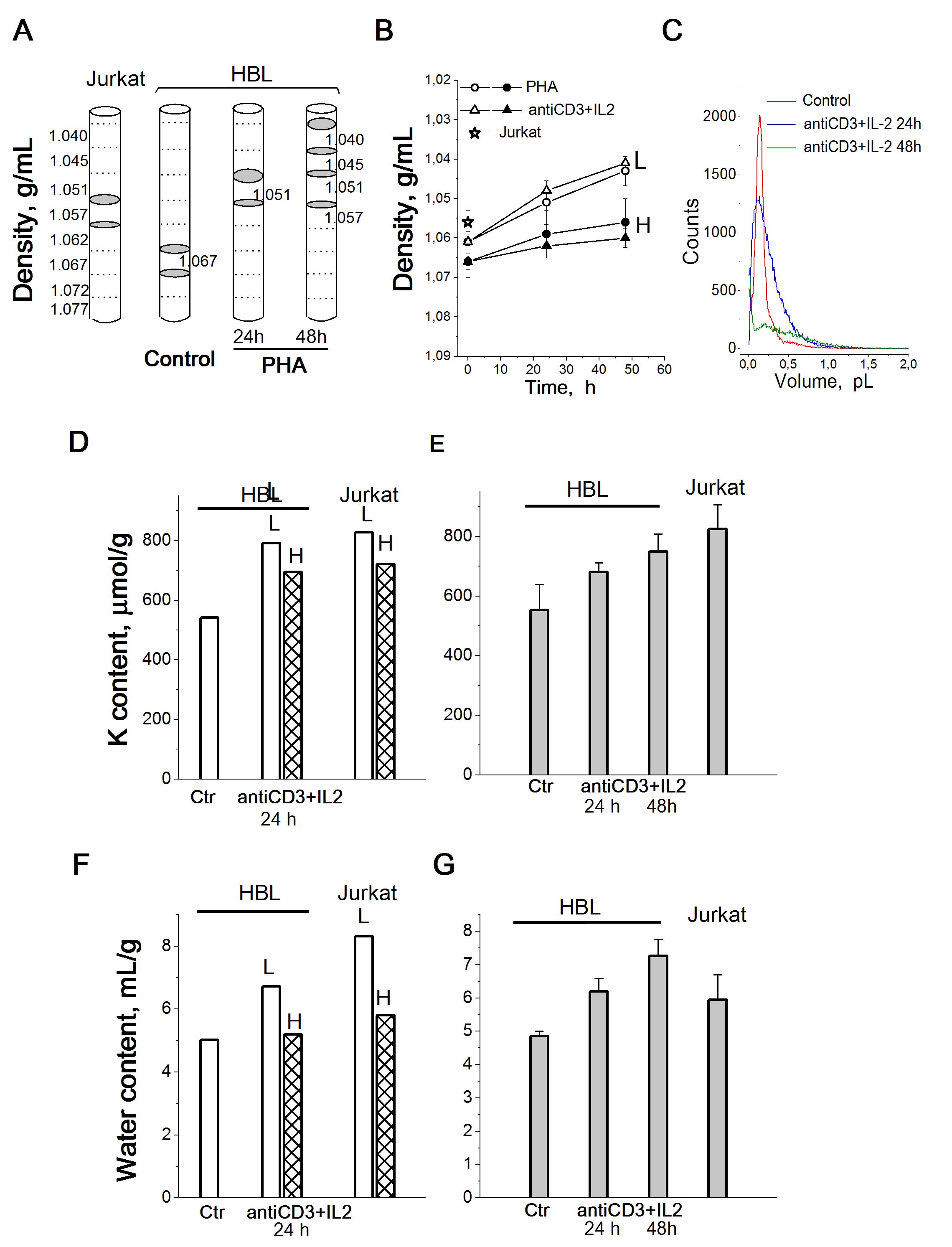
Figure 6. Cellular K+ and water content in HBL with different buoyant densities compared to unfractionated activated HBL and cycling T cells Jurkat. A: Schematic illustration of cell distribution in a discontinuous Percoll gradient. Grey areas indicate the location of T cells Jurkat, resting and PHA-activated HBL isolated for analysis. B: Cell density decreases in HBL stimulated from quiescence to proliferation. L – light and H - heavy cell populations. C: Representative cell volume distributions obtained using a Scepter Counter for HBL, resting and stimulated by (anti-CD3+IL-2) mixture for 24 and 48 hours. Control - resting HBL. D, F: Cellular K+ (D) and water content (F) in light (L) and heavy (H) cell populations stimulated with anti-CD3 with IL-2 and in proliferating T cells Jurkat. E, G: Cellular K+ (E) and water content (G) in total unfractionated HBL stimulated with anti-CD3 with IL-2 and in proliferating T cells Jurkat. One representative experiment performed triplicate. Data are means±SD. According to [24].
Here we reviewed evidence suggesting that intracellular K+ may be involved in the regulation of cell proliferation as the dominant intracellular osmolyte that controls cell volume changes associated with cell growth during the transition from quiescent state to cell cycle and continuous proliferation. An important observation in the presented studies is that an increased ratio of intracellular K+ content to protein mass occurs in cell cultures with a high proliferation rate. Relying on a theoretical analysis of the ion and water balance in animal cells [43-48], and taking into account that K+ is the main cation compensating intracellular anions we assume that a higher ratio of K+ content to protein can be interpreted as higher water content in cycling cells compared to quiescent cells.
It should be noted that the significance of K+ for maintaining cell homeostasis and viability is in complete agreement with data on the role of monovalent ions in apoptosis [9, 52-56]. The loss of intracellular K+ is a critical component of apoptosis, programmed cell death which is usually accompanied by a decrease in cell volume. Determination of the content of water and ions in apoptotic Ehrlich ascites tumour cells [57], as well as in U937 cells induced to apoptosis by staurosporine [58-60], indicates the active role of intracellular K+ as the main regulator of cell volume responsible for the apoptotic volume decrease.
The functional significance of changes in cell hydration has not been studied. Higher water content may be important for the "biochemical life" of a cycling cell. There is evidence that the water content of cancer cells is similar to that of embryonic tissue, but consistently higher than that of normal cells of similar origin, and increased cell hydration has been proposed as an important factor in carcinogenesis [61]. Measurements of intracellular water in erythrocytes of different ages suggested that cellular water loss may underlie the erythrocyte aging [62]. Cells tightly maintain the cytoplasmic concentration of macromolecules since it is directly coupled to the cell volume and impacts on macromolecular crowding. Recent studies using quantitative Raman microscopy demonstrate that under the same physiological conditions, the concentrations of cellular proteins, lipids, and water are maintained within a narrow range and change in different physiological states, such as stages of the cell cycle, attachment to substrates, or entering senescence [63]. It is assumed that the accumulation of macromolecules may play a role in the signaling associated with cell volume changes [64-66]. The relationship between cell hydration and proliferation may reflect the effect of macromolecule accumulation on cell metabolism and gene expression [67-72].
Experiments were performed using facilities of the shared research facility “Vertebrate cell culture collection” supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-683). The authors are grateful to the researchers of the Department of Intracellular Signalling and Transport of the Institute of Cytology RAS in Saint-Petersburg for providing the MSC cell lines and comprehensive support of the work.
Author contributions
Conceptualization, A.A.V., I.I.M.; investigation, I.I.M., V.E.Y.; writing, original draft preparation, A.A.V., I.I.M., V.E.Y.; I.I.M. V.E.Y. approved the submitted version.
FundingThe research was supported by the State assignment of the Ministry of Science and Higher Education of Russian Federation (075-03-2023-621).
The authors have no conflicts of interest to declare.